Table of Contents
Take a glass-stoppered retort with a capacity of about 250 c.c. and a long beak; clean and dry it. Weigh out 25 to 30 grams of potassium nitrate and transfer it to the retort through either a glass funnel or an improvised paper funnel, taking care that none of the salt adheres to the neck of the retort. Fix up your retort and receiver as shown in fig. 26; calculate how much sulphuric acid will be required according to the following equation :
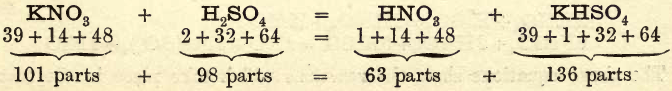
to decompose the potassium nitrate, add it to the contents of the retort and replace the stopper. Gently heat the retort; after a short time you will observe brown fumes rising and drops of liquid forming on the sides of the beak, and running slowly down into the receiver. Keep the receiver cool by allowing water to drop on it as shown in fig. 26.
The liquid in the receiver, as it accumulates, will be seen to be brownish yellow, heavy, and fuming when exposed to the air. This is concentrated nitric acid.
Nitric Acid Gas Laboratory Experiment I
Pour a drop or two of this acid into a test-tube, add a little water, then a small strip of copper foil; a violent action follows, a dark reddish-brown gas is given off, and the liquid becomes a greenish colour.
8HNO3 + 3Cu = 2NO + 3Cu(NO3) + 4H2O
2NO + O2 = 2NO2 (reddish-brown gas)
The NO is a colourless gas, as will be seen later on ; but coming in contact with the air, it takes up oxygen and forms NO2.
This is one test for nitric acid.
Nitric Acid Gas Laboratory Experiment II
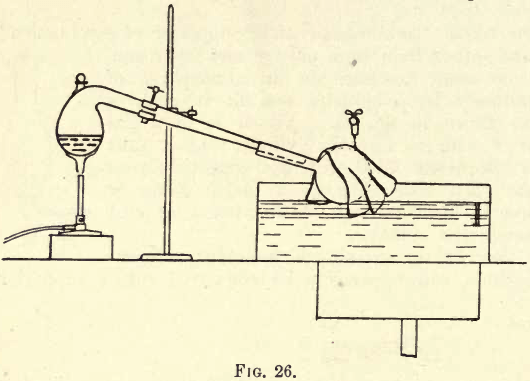
Dissolve a few crystals of ferrous sulphate in a little cold water in a test-tube. Into the test-tube used in Exp. I. pour a few drops of nitric acid to cause a fresh evolution of gas. Hold the two test-tubes so that the ruddy fumes may fall into that containing the ferrous sulphate solution, as shown in fig. 27 ; the clear solution will become rapidly darkened in colour, until it is nearly black. This darkening effect of the oxides of nitrogen, when brought into contact with ferrous sulphate solution in the cold, is another and most delicate test for nitric acid. If you boil the dark solution the colour will be almost all discharged, hence the reason for using a cold solution of ferrous sulphate.
Nitric Acid Gas Experiment III
Dissolve a little nitre in water in a test-tube, then add a few crystals of ferrous sulphate; shake the tube until all is dissolved and incline it to one side; pour carefully down the side of the tube a few drops of strong sulphuric acid, so that the heavy liquid may sink to the bottom and there form a separate layer. At the junction of the two layers a dark-brown-coloured ring will be seen, which proves the pressure of nitric acid. The dark-coloured ring is of the same nature as the dark solution of the previous experiment.
2KNO3 + H2SO4 = 2HNO3 + K2SO4
6FeSO4 + 2HNO3 + 3H2SO4 = 2NO + 3Fe2(SO4)3 + 4H2O
The above equations show the reactions which take place in the test-tube.
The sulphuric acid acts upon the nitre, forming nitric acid and potassium sulphate; part of the ferrous sulphate is acted upon by the nitric acid, forming ferric sulphate and lower oxides of nitrogen; the latter dissolve in the excess of ferrous sulphate, and give the dark brownish-black colour.
In this experiment care must be taken to keep the solution cool.
