Table of Contents
Non-swelling calcium montmorillonite is mined from bentonite deposits and treated to produced acid-activated powders and granules for use as separation products and catalysts. In particular, acid-activated montmorillonites are used as sorbents for removing impurities from fats and oils, as catalysts for mineral oil processing and for a number of other applications. These smectite group minerals are mined and manufactured around the world and new uses are continually being found for these materials.
Acid-activated montmorillonites often are used as adsorbents because of their exceptional capacity to adsorb impurities and are used principally for the purification and modification of organic chemicals. Acid-activated montmorillonites are also particularly useful as catalysts because of their large pore size and surface acidity that offers unique properties in strong acid-catalyzed reactions. These features allow acid-activated montmorillonite to be used in a large variety of applications including fats and oils bleaching, mineral oil purification in contact / filtration processes, as fixed bed adsorbents and in catalytic applications. And, the presence of acid-activated montmorillonite is growing to include new uses. Recently published articles or patents expand on the above but also detail novel uses for acid-activated montmorillonite including: manufacture of amines, production of organopolysiloxanes, detergents, agents to contain nuclear waste, precursors to pillared clays and many other user-specific applications.
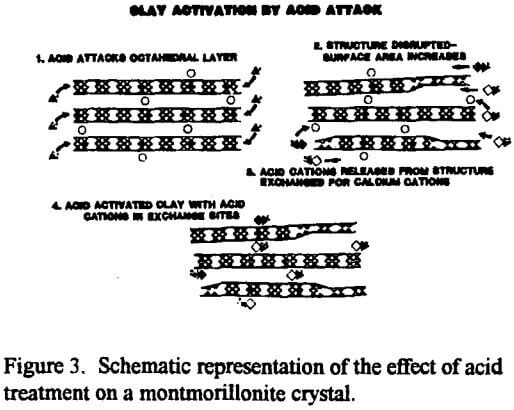
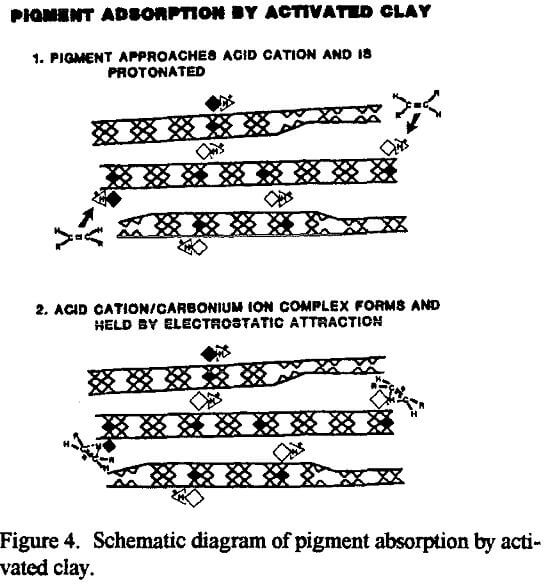
The exposure of the Al3+ and Mg2+ cations by acid activation enables the catalytic or sorptive process to proceed. In a sorption process, for example, when a polar or semipolar impurity in an otherwise nonpolar medium comes in contact with one of the active sites, Al3+ and Mg2+ cations in the interlayer gallery or the exfoliated octahedral sheet, the impurity can be attracted to and bond with one of the active, acidic inorganic interlayer cations. Now anchored in place by electrostatic forces, the captive molecule will be removed from the bulk of the liquid along with the clay by filtration.
The surface area, surface acidity and acid site concentration change as the severity of the acid treatment is increased. Surface area and bleaching efficiency (specific ability to adsorb colored pigment molecules) increase with additional acid treatment to a maximum then decrease with additional treatment. Continued acid treatment causes the structure to collapse upon itself, rendering a portion of the original surface area inaccessible. In contrast, the cation exchange only declines with additional acid treatments because the solubilized aluminum and magnesium cations coming from the octahedral layer reduce the charge disparity caused originally by the atomic substitutions in that layer.
Specifically, each Mg2+ in the octahedral layer must have its single charge deficiency balanced out by a charge from the interlayer cation, typically a Na+ or half a Ca2+. Eventually, continued acid treatment would dissolve all the Mg2+ from the octahedral layer, leaving no “unbalanced” charge to be balanced by the interlayer (exchangeable) cations. At that point, the remaining “solid” material is virtually all silica, and the cation exchange capacity (CEC), and the adsorptive capacity, would be about zero.
Edible oils
The production of edible oils involves refining, decolorizing, purifying, and stabilizing vegetable or animal oils and fats. These processes all entail the removal of contaminants that can hinder the oil’s quality and stability.
Treatment of an oil with acid-activated montmorillonite is an integral part of a multiple step process. Raw vegetable or animal fats or oils are first treated (refined) in aqueous two-phase systems to separate out water-soluble impurities such as phosphatides, fatty acids, gums, and trace metals. Acid clay bleaching removes oil-soluble impurities by sorption and acid-catalyzed reactions. After removal of the spent clay by filtration, the bleached oil can be deodorized under vacuum to remove volatile off flavors and fatty acids. Finally, the deodorized oil can be hydrogenated to increase its solidity / crystallinity. The bleaching process in particular enhances the finished product’s resistance to color reversion, fatty acid formation and oxidation and thereby improves its long term stability and shelf life.
Refined vegetable oils typically contain two major classes of pigments: chlorophyll, which causes the oil to appear green, and carotenes which impart a reddish-orange tint. Chlorophyll is considered to be the most important color to be removed for US consumer products. The amount of chlorophyll originally contained in the refined oil can be affected by harvesting conditions and growth of the vegetables. Also, the same type of oil from different regions may need different types of acid active sorbents for manufacture of a salable product. In addition to color bodies, acid-activated montmorillonite will also adsorb and remove phospholipids, some catalyst poisons, soaps, metals and oxidation products (Patterson, 1992).
Typical process conditions for use of these clays is to mix an amount of acid-activated clay, usually 0.5 to 4% by weight, with the oil for about 30 minutes at 80-120°C. This process is typically carried out with a continuous slurry contact system followed by filtration on alternating filters. The spent clay is normally landfilled, although some is burned for its residual (~30%) oil content. In addition, intensive efforts are being made to try to recover and recycle the acid clay, with limited success and virtually no commercialization so far.

Petroleum applications
Acid-activated montmorillonites are used in a number of petrochemical purification applications including the purification of BTX (benzene, toluene and xylene), cumene, mixed xylene products and jet fuel. In these applications, acid-activated montmorillonite is used catalytically to remove olefins by solid-acid-catalyzed alkylation, to decolorize the material by reaction or sorption, and, if needed, to purify the oil by removing impurities such as fats, waxes and paraffins. BTX is a mixture of aromatics derived from catalytic reforming, hydrodealkylation, and naphtha cracking. In the manufacture of this product, the removal of trace olefins is a key manufacturing step and the active sites in acid-treated montmorillonite are particularly well-suited for this application. Also, acid-activated montmorillonite removes color bodies so that the refined product has a clear, non-cloudy appearance.
Recycling of used motor oil is relatively recent use for acid-activated montmorillonite. In this application, the removal of color bodies, metal and degradation products is the key function of the acid-activated montmorillonite. After separation of the spent clay by filtration or decantation, the recycled oil can be used as a blend in making a new oil product. Spent clay from these applications is usually neutralized and landfilled, although here again the residual oil content renders it suitable for burning in a contained process.
Catalytic applications
As catalysts, acid-activated montmorillonite clays are unique in that they possess not only high solid-acid properties for acid catalyzed reactions, but also large regular pore openings in the range of 20 Angstroms. This latter feature is not found in zeolites or molecular sieve catalysts. This combination permits acid-activated montmorillonite to be used in various catalytic applications such as the manufacture of silicone oils used in sealants where the clay acts as a polymerization agent or for the manufacture of polyesters where it acts as an oligomerization agent. Acid-activated montmorillonite is also used as a catalyst in esterification, dehydration, and alkylation processes.
Other applications
The patent, technical and commercial literature also discloses the use of acid-activated montmorillonite in a great variety of other applications. In the production of sulfur, it is used to refine and decolorize. It is widely used in the beverage industry for the clarification and stabilization of beer, wine, syrup and juices. In the paper industry, it is used as a collect for pitch and as a developer for carbon-less copy paper. Other applications include use in water purification systems, as carriers for insecticides and fungicides, as a dehydration agent, as an ingredient in fire extinguishing systems, and in detergent and cleaning applications.
