Table of Contents
- History and Commercial Examples of In-Situ Copper Leaching Methods
- Copper Deposits
- Chemistry of Leaching Copper
- The Effect of Bacteria on the Solubility of Copper Minerals and the Reaction of Bacteria to Radioactivity
- Mechanics of Leaching
- Nuclear Explosives
- Public Liability
- Safety Problems
- Application of In-Situ Leaching to Nuclear Fractured Deposit
- Economics of Nuclear Fracturing and In-Situ Copper Leaching
The copper in many large low-grade and small high-grade deposits cannot be economically recovered by conventional methods because the value of the copper content of the large low-grade deposits is less than the cost of recovery, and the tonnage in the small deposits is not enough to amortize a recovery plant. These deposits are suitable targets for nuclear fracturing. Fracturing of such deposits with a nuclear explosive will permit circulation of solutions through the rock; and recovery of the copper at a cost estimated to be lower than by conventional methods which require mining, concentrating, smelting, and refining.
It should not be inferred that the use of nuclear explosives would be necessarily confined to low-grade or small ore bodies. Some of the ore bodies presently minable by conventional methods and containing copper in leachable minerals such as chalcocite, bornite, covellite, the oxide minerals, and even chalcopyrite may be more economically mined by fracturing with nuclear explosives and subsequent leaching than by conventional methods. Some deposits may be mined by conventional methods to a point beyond which the operation is economic then converted to nuclear fracturing and in-situ leaching methods.
The development of any new method or process progresses systematically from an idea through laboratory tests, bench tests, and pilot plant tests, to a full-scale operating test. Nuclear explosives are not adapted to small-scale test work. Much laboratory and analytical work has been completed, and nuclear explosive fracturing followed by in-situ leaching appears to be a practical method for recovering copper. However, many combinations of factors are involved, and the method can be proved effective and economical only by a full-scale test in a copper deposit.
Some points that can be determined only by a full-scale test are the percolation rate of solutions through the fractured rock, the copper recovery rate, the extent to which radiation safety measures will influence costs, and the time required, if any, for the decay of radioactivity to reach a level that will permit safe leaching.
Indicated advantages over conventional methods of recovering copper are the lower cost and the shorter time interval from inception to production. The method may be applicable and may prove economic when used on very low- grade deposits and thus greatly increase copper reserve.
Nuclear explosive fracturing followed by in-situ leaching is proposed as a method for the recovery of copper; it is assumed, and present information indicates, that control of radiation hazards, including any contamination of the copper produced, will be feasible. Tests are currently in progress to confirm that these hazards will be controllable, both from the operational and the cost viewpoints. Complete evaluation of the method, including economics, can be made only after a full scale test has been completed.
This Bureau of Mines report describes copper deposits, current methods of recovering copper by percolation leaching, and methods of fracturing rock with a nuclear explosive. It considers the application of current leaching methods to copper deposits that have been broken and fractured with a nuclear explosive. A brief resume of the extent of the copper industry and the recognized copper reserve of the United States is included. The mineralization of copper deposits; available information on the solubility of copper minerals, chemistry of leaching copper, and mechanics of leaching copper are described in sufficient detail to serve as a guide for the laboratory study of potential safety problems that may be induced when the deposit is subjected to the radioactivity of a nuclear explosion.
Research by the U.S. Atomic Energy Commission (AEC) and various contractor agencies under the Plowshare Program has indicated that large masses of rock can be fractured safely by the use of nuclear explosives. Cooperative research by the AEC and the Bureau of Mines has indicated that fracturing a deposit containing leachable copper minerals with nuclear explosives will permit recovery of the copper by in-situ leaching methods.
The term “in situ” is used in this report in preference to the more ambiguous term “in place” to designate ore that may have been broken or fractured but otherwise has not been removed from its original geologic location by the process of recovering the copper. Ore in dumps as well as in situ is said to be leached in place by the copper industry in contrast to ore that is crushed, ground, pulped, and said to be leached in process.
Recovering copper by leaching rock in situ is not new. However, it can be done only when rock is broken and fractured so that solvent solutions can be percolated through to contact the copper minerals. The method has been successfully used after mining was discontinued at block-caving mines where large masses of rock containing copper minerals were broken and left in the mine. Before the advent of nuclear explosives, there was no method of breaking and fracturing large masses of rock except by large-scale mining methods. These would not be economical if used solely to prepare a low-grade copper deposit for in-situ leaching, but a deposit could be fractured economically and safely with nuclear explosives.
Cost reduction in the United States has resulted from mining and metallurgical research leading to more efficient extraction and beneficiation of copper ores. Research on any method that gives promise of reducing the cost of producing copper and making available low-grade reserves not presently economic is well justified by the increasing demand for this vital metal. The successful application of nuclear fracturing and in-situ leaching may substantially increase the copper reserve and lower the cost of copper to American industry. The method eliminates the time consuming and costly construction of concentrating and smelting plants to process large tonnages of rock and may provide a method of expanding copper production rapidly in times of emergency.
Details on the formation, description, and phenomenology of nuclear tests are described and a bibliography has been compiled and published by the U. S. Atomic Energy Commission. Information has been used freely from these publications.
Information has been used from numerous reports by the Bureau of Mines on various phases of copper leaching and processing. Some have been listed as footnotes. The reader is referred to the general list of publications by the Bureau of Mines, U. S. Department of the Interior, for a complete listing.
History and Commercial Examples of In-Situ Copper Leaching Methods
A rapid growth in the use of copper has occurred in the last 150 years as a result of the development of electric power and the expansion of the brass and bronze industry. During this period world demand for copper has expanded from 10,000 tons to more than 5 million tons per year. The copper mining, smelting, refining, and fabricating industries throughout the world have expanded rapidly in the past half century.
About 1940, the United States shifted from its position as the world’s largest exporting country to that of the world’s largest importing country, at the same time continuing to be the world’s largest producer as well as consumer of copper. The copper position of the United States, on the basis of current mine output and apparent low cost of production, ranks first. However, on the basis of grade and extent, the United States copper reserve may be third. Huge African reserves are reported to contain 3 to 6 percent, Chilean reserves are reported to exceed 2 percent, while the reserves of the United States average less than 1 percent copper. Known domestic copper ore reserves that might be mined at present copper prices probably will be exhausted in 30 years or less. However, copper ores of progressively lower grades probably will continue to be mined, and more emphasis may be placed on in-situ leaching as a method of recovery.
The method of recovering copper from ore by acid leaching first was used in ancient times, probably in Cyprus, Armenia, Egypt, and other places. Agricola described methods used to recover vitrol and copper from ore in the 15th Century. Dump or heap leaching in Europe began in Germany in the 16th Century and reached great efficiency in the Rio Tinto mine in Spain where it is still used.
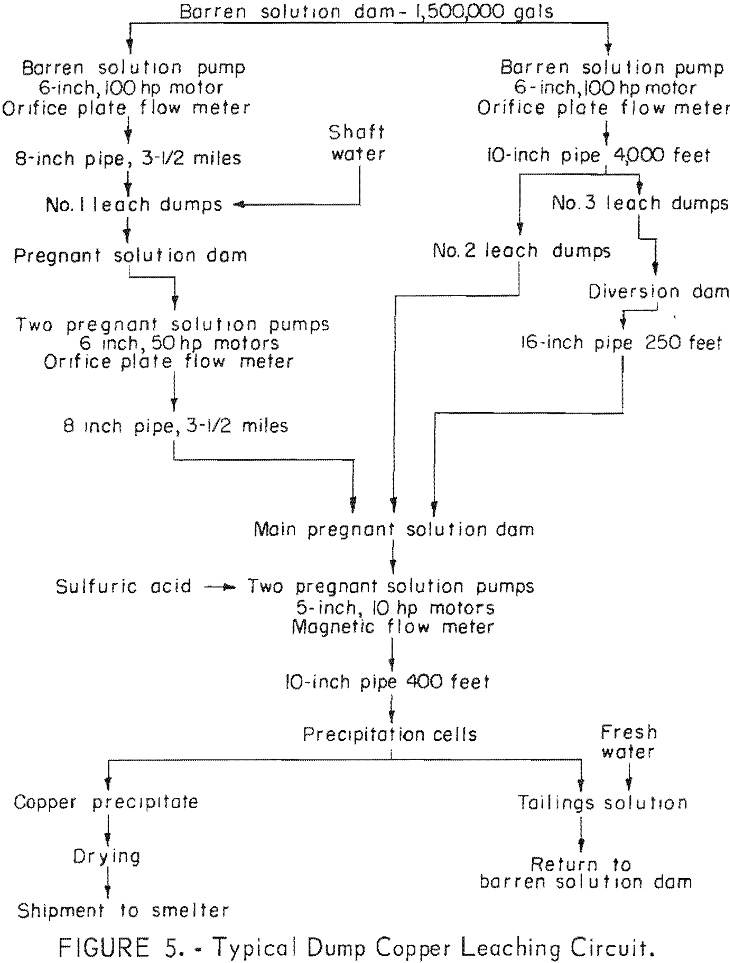
In the United States, the precipitation of copper from mine waters began about 1890 at Butte, Mont., and has been used at various localities. The first serious attempt to apply heap (dump) leaching to copper ores in the United States was made by Phelps Dodge Corp. about 1900, after James S. Douglas visited Rio Tinto in Spain and was impressed with the possibilities of the process for Bisbee ore. Subsequently the method was used on many of the dumps from large open-pit copper mines. Many mining plants now incorporate dump leaching as an integral part of the copper recovery system (fig. 1).
The first significant application of the leaching method to rock in situ was at the Ohio copper mine, Utah, in 1922. Previous to this time, inoperative mines in the Butte district had been flooded and the dissolved copper recovered from solutions. After mining by the block-caving method was terminated at the Ohio copper mine, an inverted cone of broken rock containing about 38 million tons remained. Water was applied to the top of the broken rock, percolated down through the copper-bearing rock and collected in an adit below. The copper was recovered in a successful leaching operation. After the success at the Ohio copper mine, in-situ leaching was generally attempted and often succeeded on large masses of broken copper-bearing rock that remained after large-scale mining operations were terminated.
After considerable areas of copper-bearing ore had been developed in the Butte district of Montana and many stopes filled with waste containing some copper sulfide, copper sulfate began to appear in the mine water. After years of underground work, extensive areas of stope, drift, and other underground workings were opened, and these became filled with waste containing some copper minerals. The copper minerals were oxidized by exposure to the air, which made them more soluble in the mine water and increased the copper content.
In November 1889, water was channeled into the St. Lawrence mine in an effort to extinguish a fire. When this water was pumped from the mine it was rich in copper sulfate and the first production of copper from mine water resulted. Some time after 1890, the Anaconda Company began operating a precipitating plant and has produced copper from the mine water ever since.
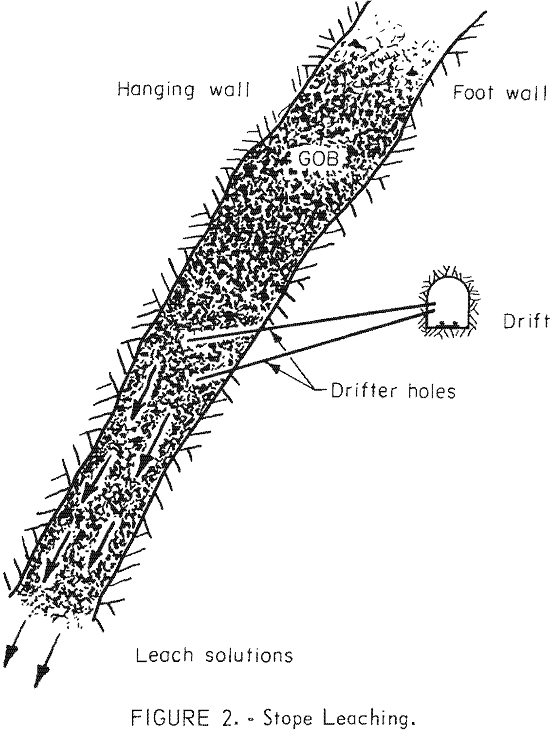
Special effort is made to bring the acid mine water in contact with old filled stopes. Where possible, parts of the mine are flooded then the water is pumped to the surface and passed through a copper precipitating plant. Water is introduced into old filled stopes by drilling long holes with a percussion drill to intersect the stopes from lateral drifts on the footwall side parallel to the stopes (fig. 2). During some months, production of copper by leaching has yielded 1,500,000 pounds.

The open drainage in-situ method is successfully for the recovery of copper at the Miami mine of Tennessee Corp., a subdivision of Cities Service Corp., in Gila County, Ariz. During the active life of the Miami mine, about 153 million tons of ore from a deposit that from 200 to 1,000 feet in depth. The ore was mined by the block-caving method, and the subsidence resulted in a crater of about 1,500 feet in diameter and 300 feet deep. From this crater, broken rock extends downward to the mining level.
The broken rock contains portions of capping, oxide ore, crushed stope pillars, and stope ore diluted with wall rock, all of which contain some copper. Overall, the chief copper mineral is chalcocite, with chalcopyrite, bornite, covellite, malachite, azurite, chrysocolla, cuprite, and native copper as minor minerals. The copper minerals occur in seams, veinlets, and as disseminated particles in Precambrian Pinal schist and Tertiary Schultz granite. There is practically no pyrite in the capping and very little in the ore.
Solution of tail water from the copper precipitating plant and fresh water are pumped to a storage tank through Transite pipe (fig. 3). The solution discharged from the precipitating plant contains only a trace of acid; about 0.5 pound of sulfuric acid per ton of water is added. This amount is sufficient to prevent iron salts from precipitating, but does not attack the Transite pipe and storage tanks. From the storage tank, the solution is pumped to distribution centers above the caved surface where it is measured in weir boxes, and additional sulfuric acid is added to bring the strength to
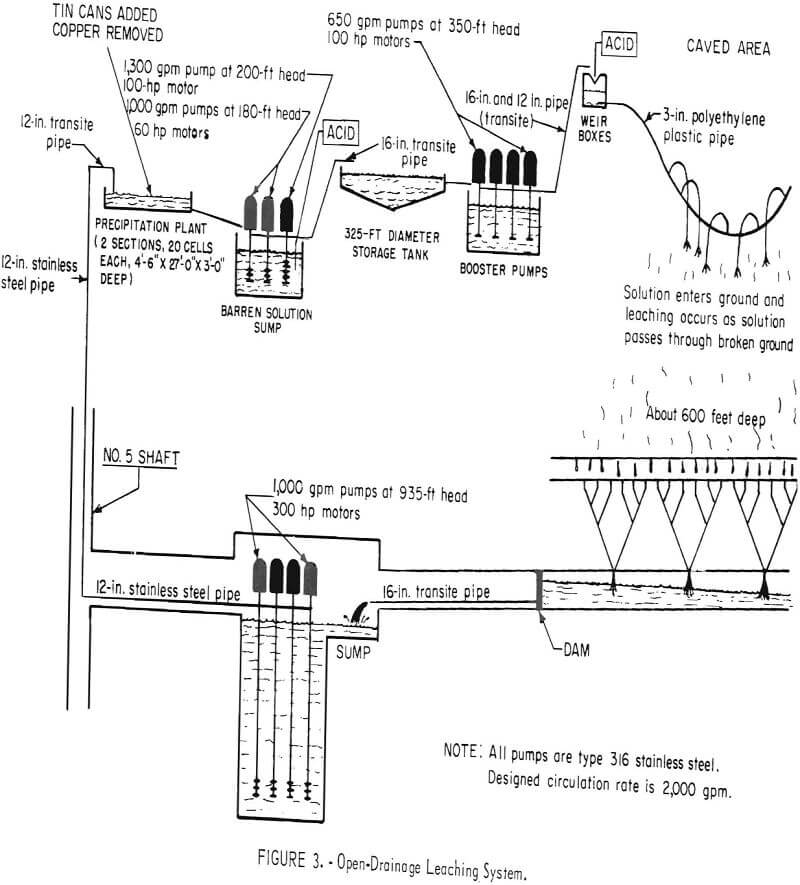
about 10 pounds per ton of water. After the acid is added, the solution flows by gravity in polyethylene plastic pipe to points of application. Ponds, drill holes, and sprays are used to introduce the solution into the ground.
The depth of the broken material averages about 600 feet. Most of the copper is believed to be in the bottom 150 feet. It requires 3 to 4 weeks after a spray is turned on the surface before solution percolates through the broken rock and comes through on the 1,000 level. The flow stops about 2 weeks after the spray is turned off. The first solution emerging is high in copper content. The spray is left in one place until the grade drops below 3 pounds of copper per ton of solution. The spray is then turned off and the area is allowed to dry. Drying and wetting periods are alternated; each new wetting brings high-grade solution but the period for which the copper content remains high decreases with repeated wettings. Many sprays are operated, some in high-grade areas and some in low-grade or partly depleted areas, and the number in each area is adjusted so that the average copper content of the recovered solution is at the desired level.
The solution is collected on the 1,000 level and pumped to a precipitation plant on the surface where it passes through cells containing detinned shredded cans. The copper precipitates as dendrites of metallic copper on the iron and is washed by high-pressure water through wooden screens. The precipitates discharge on a concrete decant slab and are drained, washed, and moved with a front-end loader to a concrete drying pad. Dried precipitates, which contain about 79 percent copper, are loaded with a front-end loader into a railroad car and shipped.
Major factors affecting the cost are the iron and sulfuric acid consumption. If the acid content of the pregnant solution becomes too high, the iron consumption in the precipitating cells is high. If the acid content of the solution pass through the broken ore is high, the consumption of acid by gangue minerals is increased. Iron salts precipitate as the solution passes through the caved material at the rate of about 4.2 pounds of iron per ton of solution. At Miami the acid strength is kept below 15 pounds per ton of solution and the consumption is 2.4 pounds of acid per pound of copper. About 1.3 pounds of scrap iron is required per pound of copper produced.
The Miami mine is operated every day of the year circulating 2,000 gpm. The pumps are operated three shifts. Underground repair crews and maintenance crews operate 5 days per week. The operation requires about 123 manshifts per week. About 9,000 tons of refined copper per year is produced from the “cement copper” precipitate.
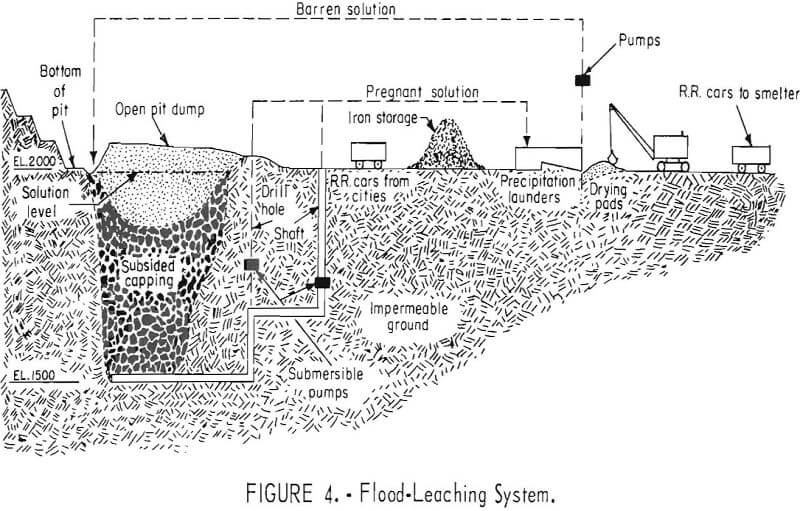
The flood-leaching method is successfully used in a combined dump and in-situ leaching operation for the recovery of copper at the Ray Mines Division of Kennecott Copper Corp, in Arizona. During the active life of the underground mine, many millions of tons of copper ore was mined from a deposit that ranged from 200 to 600 feet in depth. The ore was mined by the block- caving method, and the subsidence resulted in an elongated crater 1,000 feet wide, 2,000 feet long, and 150 feet deep. When a nearby open pit was stripped, material containing some leachable copper was used to fill this crater.
The broken rock consists of portions of capping; oxide ore, crushed stope pillars, diluted stope fill, and stripping from the nearby open pit. Most copper occurs in the minerals chalcocite, chalcopyrite, and cupeuferous pyrite. Malachite, azurite, chrysocolla, dioptase, cuprite, and native copper occur in the capping. The ore minerals are in seams, veinlets, and disseminated particles; the rock is Precambrian schist, diabase, and monzonite porphyry. Pyrite is plentiful in the primary ore, and some is intermingled with the chalcocite.
The bottom of the ore is well below ground-water level, and the rock surrounding the broken ore in workings is tight and impermeable. The bottom level is at 1,500 feet above sea level and the bottom level of the adjoining open pit is at 2,000 feet above sea level. After the mine was closed, the workings were flooded to the 2,000-foot level. Water, obtained from the gravels of Mineral Creek, is in short supply during part of the year. During this dry period, the water level may be lower than the 2,000-foot level, thus exposing the material to oxygen. Dissolved sulfates and oxidizing pyrite and other sulfides provide abundant acid.
Copper-bearing solution is pumped from the flooded mine at three points by submersible pumps in shafts and drill holes connected with the underground workings. About 2,000 gallons per minute is pumped and passed through a precipitation plant where the copper is removed. Spent solution is returned to the flooded mine. No attempt is made to distribute the return water; it is returned to a sump on the surface distant from points at which pregnant solution is withdrawn. It again becomes charged with dissolved copper by diffusion as it percolates through the broken rock (fig. 4). In addition to the flood-leaching operation in the abandoned underground mines, open-drainage dump leaching is used to recover copper from low-grade material in the open-pit waste dumps.
Copper Deposits
Most copper has been deposited in the earth’s crust as a sulfide mineral by solutions in channels through which the mineral-bearing solutions escaped from the interior of the earth. Sometimes the path of the solution was a definite, confined, and comparatively narrow fracture or pipe, and the minerals were deposited in this channel or in connected cavities to form a high-grade deposit. At other times the channel was a wide zone containing many small fractures, and the copper minerals were deposited in small seams, fractures, cavities and as disseminated particles in the rock over a wide area to form a low-grade but extensive deposit.
Weathering processes, either in the present or former geologic periods, have altered the near-surface sulfide minerals to oxide, carbonate, silicate, or sulfate minerals. Sometimes, after the oxide minerals are formed, geological processes have covered the deposit with sedimentary or volcanic rock. Thus, although generally formed near the surface, deposits of oxide copper minerals may occur at great depths.
The weathering process continues in deposits where sulfide copper minerals are exposed to air and moisture. Soluble copper minerals are dissolved from the upper oxidized part of the deposit by rain water, and the solutions migrate downward. Copper is removed from the rock near the surface, and a blanket of leached capping is formed. The copper content of the underlying sulfide zone, when compared to the copper content of this leached capping, may be a measure of the amount of copper that is recoverable from a sulfide deposit by in-situ leaching.
When the solutions reach permanent water level, the hydrogen-ion concentration increases because of the inaccessibility to oxygen and slight ionizing of sulfide material. The copper is precipitated from the downward migrating solution as the minerals chalcocite, covellite, or bornite, thus forming a zone where secondary sulfide copper minerals intermingle with and enrich the primary sulfide minerals. This enriched zone often consists of copper ore that ranges from 200 to 600 feet or more in thickness and forms deposits that sometimes exceed a billion tons. Deposits of this type are the source of most of the copper ore that is now mined.
Copper minerals are widely distributed in the surface of the earth. Rock that contains copper minerals is classed as ore when copper is in a recoverable form and its recovery is profitable. The cost of mining and processing the ore including finding the deposit and recovering, refining, and marketing the copper must be less than the sale value of the copper recovered.
Large deposits of copper-bearing rock become ore when the selling price increases more rapidly than the cost of recovering the copper or when a new extraction method is developed that will reduce the cost of production, as the proposed use of nuclear fracturing and in-situ leaching may do.
In copper mining the term “oxide ore” indicates rock containing combinations of oxide, carbonate, silicate, sulfate, and sulfide copper minerals that are dissolved from a laboratory sample in a short period of time by a dilute acid; generally 5 to 10 percent sulfuric acid by volume. Copper in this form generally is not readily concentrated by the flotation method although most sulfide minerals are recovered.
The term “sulfide ore” is used by the industry to indicate rock containing those copper minerals that are not readily soluble in dilute sulfuric acid and generally can be successfully recovered by the flotation process. Native copper, recoverable by the flotation method, is usually included with the sulfide minerals. Although sulfide minerals generally are not quickly soluble in an acidic solution, most may be dissolved slowly by an oxidizing agent such as ferric sulfate, and all are soluble in long periods of time.
In 1963; 1.2 million tons of copper valued at $747 million was produced by the copper mining industry in the United States. About 81 percent of the ore and 74 percent of the 1963 copper was from open-pit mines. The average copper content of the ore was 0.75 percent. About 10 percent of the copper was recovered by leaching. The total material mined was 426 million tons of which 146.5 million tons was copper ore and the remainder was waste. Much of the waste contained a small amount of copper that eventually will be recovered by dump-leaching methods.
The Geological Survey and the Bureau of Mines published a figure of 32.5 million tons for United States copper reserves in 1959.10 Since that time more than 6.75 million tons of metal has been produced. How much of this production has been offset, or how much of the reserve has been increased by new discoveries and extensions of known ore bodies is not known.
Some industry spokesmen have quoted a reserve tonnage at least 50 percent larger than that mentioned in the 1959 release. The 32.5 million-ton copper reserve of 1959 was contained in ore estimated to average 0.9 percent or 18 pounds of copper per ton. At this average figure, the copper reserve would be contained in more than 3.6 billion tons of ore.
The 1959 reserve figures did not include metal in subeconomic deposits and mine dumps that can be leached. Large tonnages known to contain from 0.25 to 0.5 percent copper were not economic in the past nor at present, and the metal contained is not included as reserve. Reserve forecast over the past decades show an upward trend in quantity of copper available in spite of large tonnage of ore mined. This increase in reserve has resulted partly from lowering the cutoff grade as a result of improved techniques in mining and processing the ore.
Not included in the reserves are many small tonnage deposits containing as much as 2 percent copper. Such deposits contain a tonnage too small to amortize the construction of a concentrating plant and are too low grade to justify direct shipment to a smelter. Many of these deposits are contained in pipe-like form that would be ideal for nuclear fracturing and subsequent in-situ leaching.
Copper deposits containing leachable copper minerals might be successfully fractured with the use of nuclear explosives, and the copper leached from the rock in situ. Such a method of production might substantially lower the cost of producing copper from deposits of this type, thus adding them to increase copper reserves, Statistics are not available to separate leachable from nonleachable reserve and resource deposits. Until this information is available, it is difficult to predict the extent to which the proposed method may be applicable.
Leaching is the term applied to the process of recovering valuable minerals or metals from an ore by dissolution leaving the gangue or waste material virtually unaffected. Subsequent recovery of the metal or metals from solution is effected through either chemical or electrolytic precipitation.
An important factor in determining the leachability of a copper deposit is the type of host rock in which the minerals are deposited. Copper is found in rock types ranging from carbonate to silicate in sedimentary, metamorphic, or igneous formations. Some deposits are in carbonate rocks and cannot be leached economically with acid because the gangue consumes excess amounts of acid. Some crystalline rocks that are hosts for disseminated copper deposits are diabase, monzonite, granite, tactite, argillite, hornfel, and schist. Many deposits have few or no acid-consuming minerals in the associated gangue; these may be suitable for nuclear fracturing and in-situ leaching. Next to percentage of copper extraction, the solvent consumption of the gangue rock is the most important item investigated in the examination of a deposit for possible in-situ leaching. The copper extraction and acid consumption of the rock can be approximated from a metallurgical test.
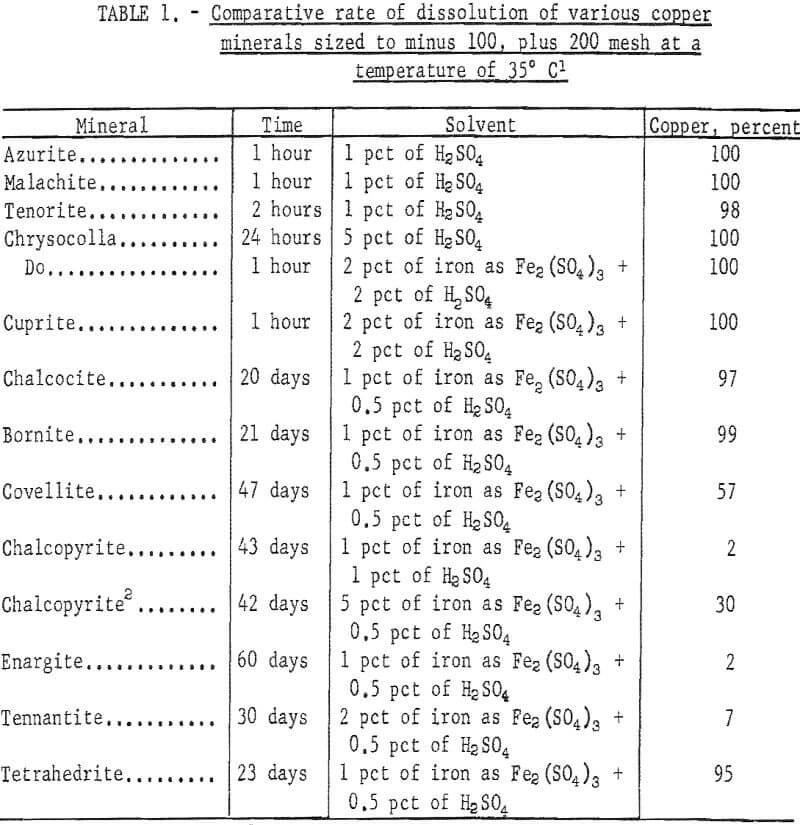
Copper minerals are oxidized and may be dissolved eventually in geologic periods of time. Before the perfection of the flotation method, research was directed toward the determination of solvent and mechanics for the rapid dissolution of sulfide copper minerals. Efforts were pointed toward determining the solubility of copper minerals in terms of hours and days. More research is needed to determine the rate of dissolution of the more difficult soluble copper sulfide minerals in periods of months and years.
Oxide minerals are readily soluble: simple sulfide minerals chalcocite and covellite are easily dissolved by combinations of sulfuric acid and ferric sulfate; the complex mineral bornite is dissolved with some difficulty; and chalcopyrite (CuFeS2) is dissolved in a short time, with extreme difficulty; it is often considered nonleachable when ore is treated in leaching tanks. The time required for dissolution of the copper minerals in a tank-leaching operation is extremely important and must be short, but time may not be so critical in an in-situ leaching project. The dissolution of a copper mineral such as a chalcopyrite at a slow rate may not preclude in-situ leaching providing the rate of acid consumption by gangue minerals is low. The amount and rate at which copper was dissolved from various minerals by Bureau of Mines tests is indicated in table 1.
Chemistry of Leaching Copper
When water and air or water containing dissolved air is percolated through broken rock containing pyrite, sulfuric acid is formed slowly according to equation 1:
2 FeS2 + 7O2 + 2 H2O → 2 H2SO4 + 2 FeSO4…………………………………………(1)
In the presence of abundant oxygen and sulfuric acid the ferrous sulfate is oxidized slowly to ferric sulfate according to equation 2:
4 FeSO4 + 2 H2SO4 + O2 → 2 Fe2(SO4)3 + 2 H2O…………………………………(2)
Ferric sulfate in the presence of sulfuric acid forms a solution that will dissolve most copper minerals. Most copper deposits contain varying amounts of pyrite associated with the copper minerals. When a deposit does not contain sufficient pyrite to form sulfuric acid the acid must be added to the leach solution. Some basic equations for the dissolution of various copper minerals are:
Tenorite, CuO:
CuO + H2SO4 → CuSO4 + H2O……………………………………(3)
Malachite, Cu2(OH)2CO3:
Cu2(OH)2CO3 + 2 H2SO4 → 2 CuSO4 + CO2 + 3 H2O………………………….(4)
Azurite, Cu3(OH)2(CO3)2:
Cu3(OH)2 (CO3)2 + 3 H2SO4 → 3 CuSO4 + 2 CO2 + 4 H2O…………………………..(5)
Chrysocolla, CuSiO3 · 2 H2O:
CuSiO3 · 2 H2O + H2SO4 → CuSO4 + SiO2 + 3 H2O……………………………….(6)
Cuprite, Cu2O:
3 Cu2O + 4 Fe2(SO4)3 → 6 CuSO4 + 6 FeSO4 + Fe2O3…………………………(7)
Covellite, CuS:
CuS + Fe2(SO4)3 → CuSO4 + 2 FeSO4 + S……………………………..(8)
Chalcocite, Cu2S (in two stages):
Cu2S + Fe2(SO4)3 → CuSO4 + 2 FeSO4 + CuS
CuS + Fe2(SO4)3 → CuSO4 + 2 FeSO4 + S……………………………………(9)
The dissolution of the double sulfides of copper and iron, bornite, Cu5FeS4, cubonite, CuFe2S4, chalmersite, CuFe2S3, and chalcopyrite, CuFeS2, is normally difficult. Bureau of Mines tests indicate that when bornite is leached with sulfuric acid and ferric sulfate, the iron is attacked preferentially and the mineral is dissolved. Tests show that with chalcopyrite the ratio of combined Cu:Fe:S in the residue remains the same as the original mineral at any stage in the dissolution and the mineral generally is dissolved very slowly.
Chalcopyrite is not soluble in an aqueous solution of sulfuric acid and is not oxidized at normal temperatures over long periods of time. It is oxidized and dissolved slowly by solutions that contain ferric sulfate or ferric chloride. Rate of dissolution may be about 1 percent per month (table 1). Sullivan and Brown studied the dissolution of chalcopyrite in 1934 and suggested equations:
CuFeS2 + 2 Fe2(SO4)3 → CuSO4 + 5 FeSO4 + 2 S…………………………………………(10)
CuFeS2 + 2 Fe2(SO4)3 + 2 H2O + 3 O2 → CuSO4 + 5 FeSO4 + 2 H2SO4…………(11)
Some leachable copper deposits have no sulfide minerals; others have abundant pyrite and sulfide copper minerals. Generally deposits containing sulfide minerals do not require the addition of sulfuric acid to the leach solution. Acid is generated when water is percolated through the broken ore. Acid must be added to the solution for those deposits that are deficient in sulfide or sulfate minerals. The amount of sulfuric acid that is added to the leach solution is shown for several operating plants on table 2.
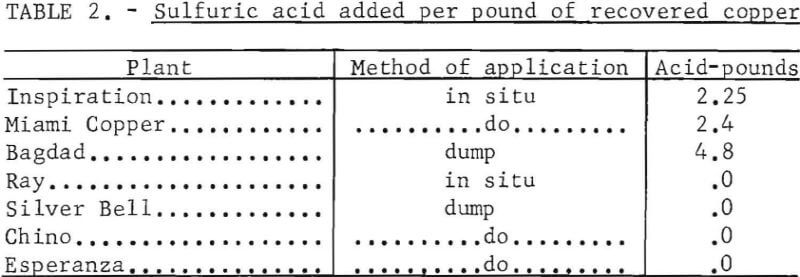
Copper has been recovered commercially from acid solution by three methods :
- Copper in solution as a sulfate is replaced by metallic iron causing the copper to precipitate.
- The copper ion in a sulfate solution is reduced by an electric current causing the copper to precipitate at the cathode.
- The acidity of the solution is reduced by addition of lime causing the copper to precipitate as a basic carbonate.
Copper in an acid solution is replaced by metallic iron according to equation 12:
CuSO4 + Fe → Cu + FeSO4………………………………………(12)
Theoretically 0.88 pounds of iron will replace 1 pound of copper. The replaced copper immediately precipitates as dendrites on the iron; however, circulating solutions contain free sulfuric acid and ferric salts in solution that also consume iron according to equations 13 and 14:
Fe + H2SO4 → FeSO4 + H2…………………………………………..(13)
Fe + Fe2(SO4)3 → 3 FeSO4…………………………………………..(14)
In actual operations the metallic iron consumed per pound of copper recovered ranges from 1.3 to 3 pounds. Good clean compact iron or mild steel will precipitate more copper than detinned cans and light iron, but presents handling and mechanical problems. Sponge iron, a porous product resulting from the chemical reduction of solid iron-bearing material at a temperature short of its melting point, is sometimes used. The iron consumed at several plants is shown on table 3.

The precipitation of copper by electric current from leach solution, called electrowinning, is accomplished by several large producers. Advantages of the method are that a refined product is produced and ferric sulfate and/or sulfuric acid is regenerated. The reaction is endothermic; electrical energy sufficient for the decomposition of CuSO4 is consumed. A solution containing copper sulfate (CuSO4) is passed through a cell containing alternate insoluble anodes and thin copper starting sheets.
If the CuSO4 is considered to ionize as Cu++ and SO4- ions, the reaction at the cathode is:
Cu++ + 2(e) → Cu………………………………………………(15)
The copper, soluble as an ion but very insoluble when neutralized, immediately precipitates as a deposit on the cathode.
At the anode the reaction is:
SO4– – 2(e) → SO4……………………………………………………………………(16)
The SO4 immediately combines with water to release oxygen and form sulfuric acid according to equation 17:
2 SO4 + 2 H2O → 2 H2SO4 + O2…………………………………………………(17)
A choice between the use of iron and electricity for copper recovery is generally made on the basis of size and duration of the operation; small and short time operations use metallic iron and large operations use the electrolytic method. Economic factors such as the cost of power and transportation are also important.
Depending on company preference, several methods are used to report the assay values of solutions: percent; grams per liter, pounds per ton, or pounds per 1,000 gallons. The flow of solutions is measured in gallons per minute. One gallon of solution weighs about 8-1/3 pounds. A flow of one gallon per minute for 24 hours is equal to 8-1/3 x 1,400/2,000 or 6 tons per day.
Copper recovery plants are designed to treat a specified amount of solution, and the copper content of the solution is adjusted to produce the desired amount of copper. The solution is circulated through high-grade (new) and low-grade (partly leached) areas of the deposit or dump. The area is controlled by the placement of the spray or solution pond. The flow is adjusted by area so that the blended flow from all areas contains the desired amount of copper. One in-situ leaching operation was designed to circulate 2,000 gpm and produce 50,000 pounds of copper per day. The calculations for grade of solution are as follows:
2,000 x 6 = 12,000 tons of solution per day.
50,000/12,000 = 4.17 pounds of copper per ton of solution
To the 4.17 pounds must be added that in the tail water (0.04) and the loss in smelting (0.07). The required copper content for feed water to the plant must then be 4.28 pounds of copper per ton of water. To assure a uniform copper content at the desired level, the outflow from the deposit is sampled. When the copper content drops below the desired amount, less solution is distributed over low-grade or partially leached areas and more over high-grade areas of the broken ore. The copper content of the solution recovered by a flood-leaching operation may be controlled by the rate of flow through the deposit.
The Effect of Bacteria on the Solubility of Copper Minerals and the Reaction of Bacteria to Radioactivity
The activities of bacteria influence the oxidation of some sulfide minerals. Water samples were collected from five Arizona copper mines and three species of oxidizing bacteria were isolated from the samples. The results of extended tests indicate that the sulfur oxidizing bacterium, Thiobacillus thiooxidans, can readily convert sulfur to acid but is not able to assimulate and oxidize the sulfur as it occurred in sulfide minerals of copper. Ferrobacillus ferrooxidans is capable of producing quantities of acid ferric sulfate from ferrous iron that dissolve significant quantities of copper from the minerals chalcocite, covellite, and bornite. The iron oxidizing bacteria Ferrobacillus ferrooxidans and Thiobacillus ferrooxidans are able to accelerate the dissolution of the iron in the minerals pyrite and chalcopyrite.
The chemistry involved in microbial dissolution of iron and copper from sulfide minerals has been described by Sutton by reference to a series of chemical equations. Pyrite in the presence of oxygen and water is slowly oxidized according to equation 1.
The iron oxidizing bacteria in the presence of oxygen and sulfuric acid then oxidize the available ferrous sulfate and, acting as a catalytic agent, accelerate the formation of ferric sulfate as shown in equation 18:
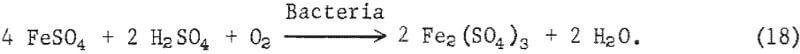
The ferric sulfate reacts with pyrite to form sulfuric acid and ferrous sulfate according to equation 19:
7 Fe2(SO4)3 + FeS2 + 8 H2O → 15 FeSO4 + 8 H2SO4………………………………..(19)
Ferric sulfate also reacts with a copper sulfide mineral, such as chalcocite, to form copper sulfate, ferrous sulfate, and elemental sulfur as shown in equation 20:
Cu2S + 2 Fe2(SO4)3 → 2 CuSO4 + 4 FeSO4 + S…………………………………..(20)
The ferrous sulfate is then reoxidized by the iron oxidizing bacteria to form more ferric sulfate, and the cycle is repeated. The elemental sulfur set free in the dissolution of the copper mineral is oxidized by the sulfur oxidizing bacteria Thiobacillus concretivoues, in the presence of oxygen and water, to form sulfuric acid as shown in equation 21:
![]()
Apparently the sulfur and iron oxidizing bacteria, by expediting the production of sulfuric acid and ferric sulfate, increase the dissolution of the copper minerals but do not attack the copper minerals directly. Basically, these bacteria do not change the nature of the chemical reaction but increase the rate of the reaction.
Investigations of bacterial leaching under conditions similar to those expected to exist in the environment of a nuclear chimney have indicated that the bacteria most important in accelerating leaching of sulfide ores can be expected to grow under these conditions. The small quantities of radioactive explosion byproducts present do not inhibit bacterial leaching. The residual heat left in the rock surrounding the nuclear explosion in most cases will tend to stimulate rather than inhibit the growth of the bacteria.
Mechanics of Leaching
Four factors are necessary for the successful leaching of copper ores; they are:
- A solution that will attack the copper minerals must be brought into contact with the particles of ore.
- The copper minerals must be dissolved by chemical action of the solution.
- The solution must find its way out of the voids of the rock and be recovered in a collecting system.
- The copper in the solution must be recovered.
Leach solutions are applied to copper-bearing rock under three circumstances in conventional practices:
- Ore is mined, crushed, and processed in a tank.
- Unsorted ore is mined, placed in a heap or dump and leached in place.
- Ore is broken or fractured and the copper is leached from the rock in situ.
There are two methods of applying solutions for percolation leaching; flood leaching (the ore is immersed in solution that percolates either up or down through the ore) and open-drainage leaching (the solution is distributed on top of the ore and collected below after percolating down through the ore).
Not classed as a leaching method is the leach-precipitate-float (LPF) system for ore containing mixed soluble and floatable copper minerals. The LPF system is actually an adaption of vat leaching to continuous processing. Acid is added to the pulp in or after the grinding section followed by finely ground metallic iron which precipitates the copper. The pulp is treated by conventional flotation to recover both sulfide copper minerals and precipitated metallic copper.
In the process of flood leaching, a solvent solution surrounds the rock and then penetrates into the body of the rock where by chemical action it dissolves the copper minerals and holds them in a soluble state. The solution around the rock has a lower concentration of dissolved copper salts than has the solution inside the rock after chemical action starts. A diffusion pressure is created and the salts diffuse from the area of higher to the area of lower concentration. After a period of time, the solution surrounding the rock is drawn and the copper removed; the rock and solution must be contained either in a vat or in a tight ground formation in order to flood the rock with solution and dissolve the copper by this method.
The ore is immersed in the solution in a tank as at inspiration or in situ as at Ray where broken rock in the caved area surrounded by a tight impervious rock is flooded with solution. The solution occupies the interstices between the ore fragments and will obey the laws of liquid flow; it tends to follow the path of least resistance among the coarse particles. The extraction of the copper from within the particles of ore is dependent on the rate of diffusion.
In the process of open-drainage leaching, the solution is applied at the top, trickles down through the ore, and, if conditions are properly controlled, is present as a film over the particles of the ore. The interstices between particles of ore are filled with air. Soluble copper salts on the surface of the rock are dissolved and carried downward and some solution enters the rock. Gravity, assisted by the cohesion of water causes the solution to pass over and through the particles of ore. The penetration of the rock is a function of time and varies with the size and type of rock. When the copper content of the recovered solution drops below an acceptable limit, the surface sprays are stopped, the solution supply is cut off, and the rock begins to dry. Capillary forces bring the contained solution and dissolved copper to the surface of the rock where the water evaporates and the soluble salts crystallize; they are easily dissolved and collected by the next application of solution. Success of the open-drainage method depends on alternate wetting and drying of the ore. Complete recovery is dependent on proper distribution of the solution over the surface and through the ore.
In contrast to the flood-leaching method, the open-drainage method does not require lateral confinement of the solution. This method is most popular in dump-leaching operations and a typical dump-leaching flowsheet is illustrated in figure 5. An impervious surface on which the solutions can be collected must underlie the ore. Increased recovery results because the film condition of the solution compels it to make contact with the particles of ore rather than to pass through void spaces, as it does when the ore is flooded. The amount of water removed from the rock during each drying period and retained during each wetting period determines the rate of extraction of the soluble copper minerals. As with the flood-leaching method, a long period of contact is necessary when large size ore lumps are being leached.
A study of the mechanism of percolation leaching comparing extractions by flood and open-drainage methods showed that alternate wetting and drying brings the copper to the surface much more rapidly than chemical diffusion. Under comparable conditions, higher and more rapid extraction was obtained by the open-drainage method in laboratory tests by the Bureau of Mines. In practice the choice between flood leaching and open-drainage leaching is based on the characteristics of the ore body and surrounding conditions. The characteristics of the ore are determined by laboratory tests, surrounding conditions by a field examination.
Nuclear Explosives
Basically, a nuclear explosive is a source of energy. Nuclear explosions are measured according to their yield. Because the amount of energy release is so great, this yield is measured in terms of kiloton units. A kiloton is defined as a prompt release of 4 x 10 9 Btu or about the same amount of energy as 1,000 tons of TNT. The nuclear device is the most compact and most powerful source of energy known to man. Because of the large amount of energy released, the cost per energy unit is low. Based on costs published by the AEC during May 1964, the cost of energy on a per unit basis is compared with conventional explosives in table 4.
Cost estimates for breaking rock with nuclear explosives have been tabulated for a scaled depth of 210 feet per kton and are shown in table 5.
In mining applications the optimum scaled depth of burst might range from 190 to 220 feet per kton depending on rock characteristics.

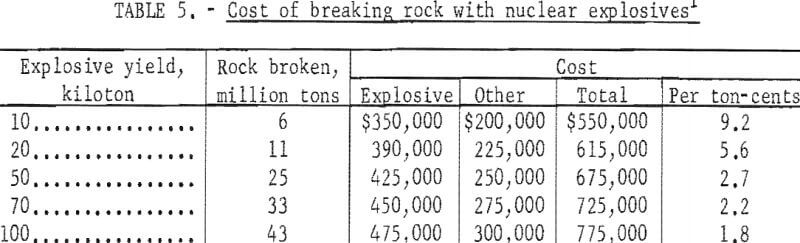
Included in these costs are: AEC charges for nuclear explosives and their arming and firing; emplacement and related engineering costs for site development; and support and other miscellaneous costs. Not included are direct costs of safety studies, which vary greatly, and are highly dependent on the region in which the project is located, and on whether a single explosion or a series is used. Projected charges for thermonuclear explosives published by the AEC are shown in figure 6.
Nuclear explosives can be designed to optimize particular characteristics such as explosive package size, cost, radioactive byproducts, and others. Of particular interest to mining is the size of the explosive. The AEC has declassified a range of yields and associated diameters of explosives that could be made available using current designs (table 6). A 5-kton nuclear explosive being lowered into a 2,700-foot hole is shown in figure 7.
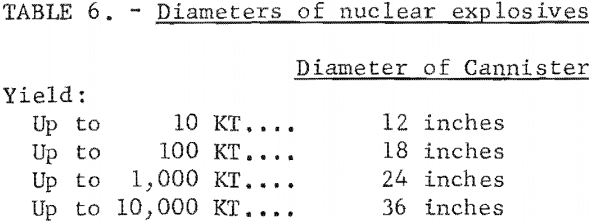
The internal diameter of the emplacement hole would have to be slightly larger, of course, to accommodate the cannister. The length of the cannister is roughly two to four times the diameter.
Underground experiments in hard rock have established the capability of nuclear explosives to break and fracture rock. More than 150 nuclear explosions have been detonated underground. The effects of a nuclear explosive

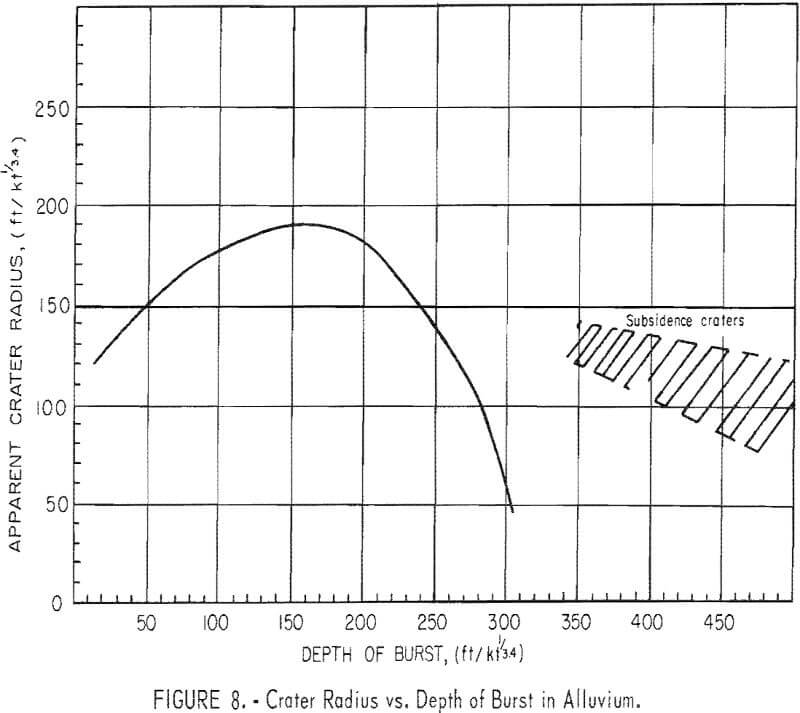
differ on the depth of burial. A factor, the scaled depth of burial, is determined by dividing the actual depth of burial in feet by the 3.4 root of the yield of the explosive in kilotons. The effects differ for different types of rock. The curve (fig. 8) shows crater radius versus scaled depth of burst for alluvium. From this type of curve, the depth of burial that will break the largest volume of rock for a yield is determined. For hard rock, this optimum rock-breaking point is slightly greater than 200 feet/kton. With this basis, the family of curves shown in figure 9 can be drawn to show tonnage data as a function of yield and depth of burst. These curves apply to cratering or intermediate nuclear explosions.
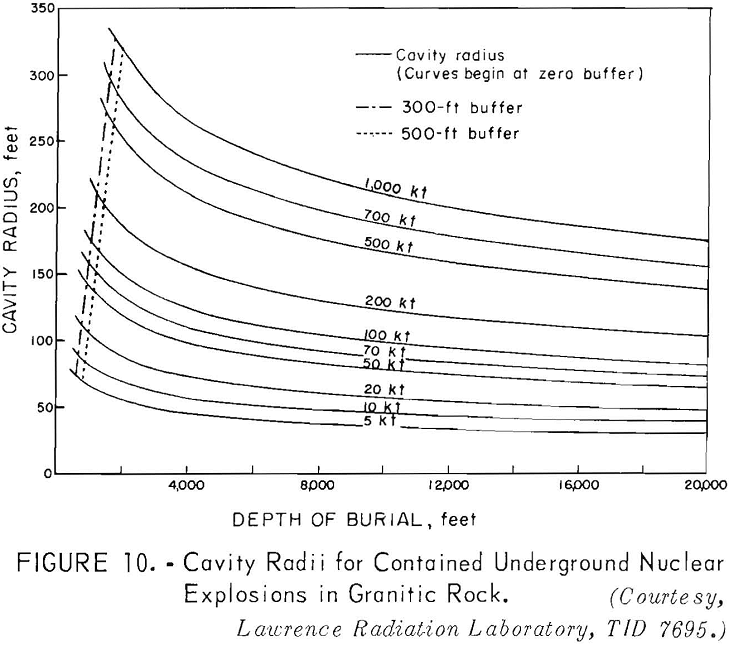
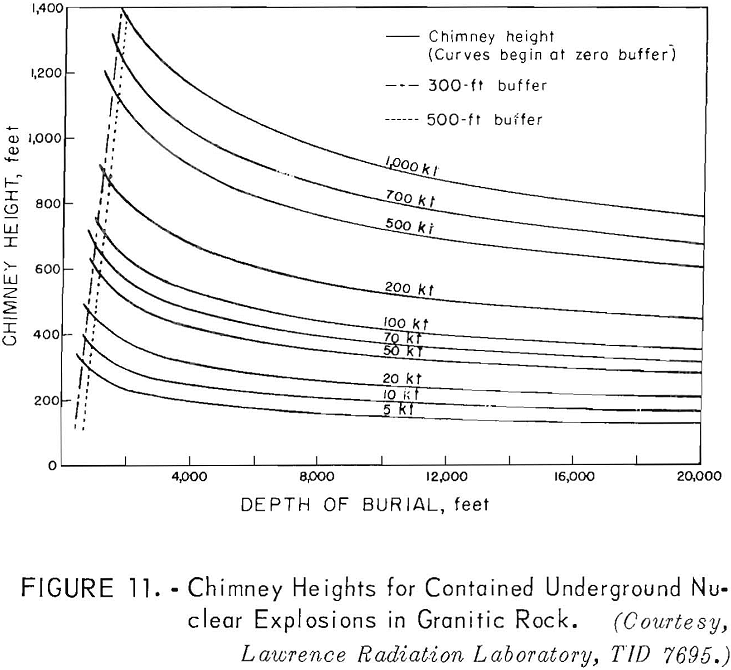
Scaling for a completely contained nuclear explosion is related to the cube root of the yield in contrast to the 3.4 root that is used for cratering and intermediate-depth explosions. Containment, as used here, means that the gross explosion effects, such as surface subsidence, the ejection of rock fragments, rubble moved, and crater formation, are prevented from developing at the surface by rock overlying the explosion; some cracks may reach the surface.
A chimney of broken rock is formed above and around the explosion center. This chimney is formed by gravity collapse of the fractured and weakened rock overlying the nuclear-explosive cavity void. Available experimental data for
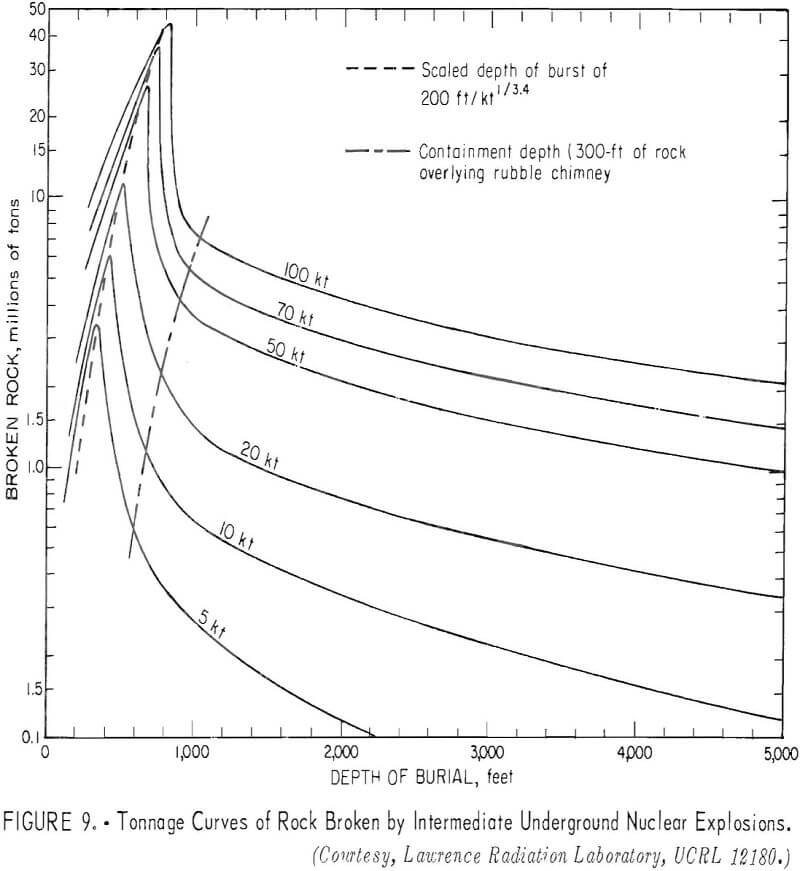
contained underground nuclear explosions permits the prediction of cavity size, chimney height; and tonnage of rock broken with considerable confidence.
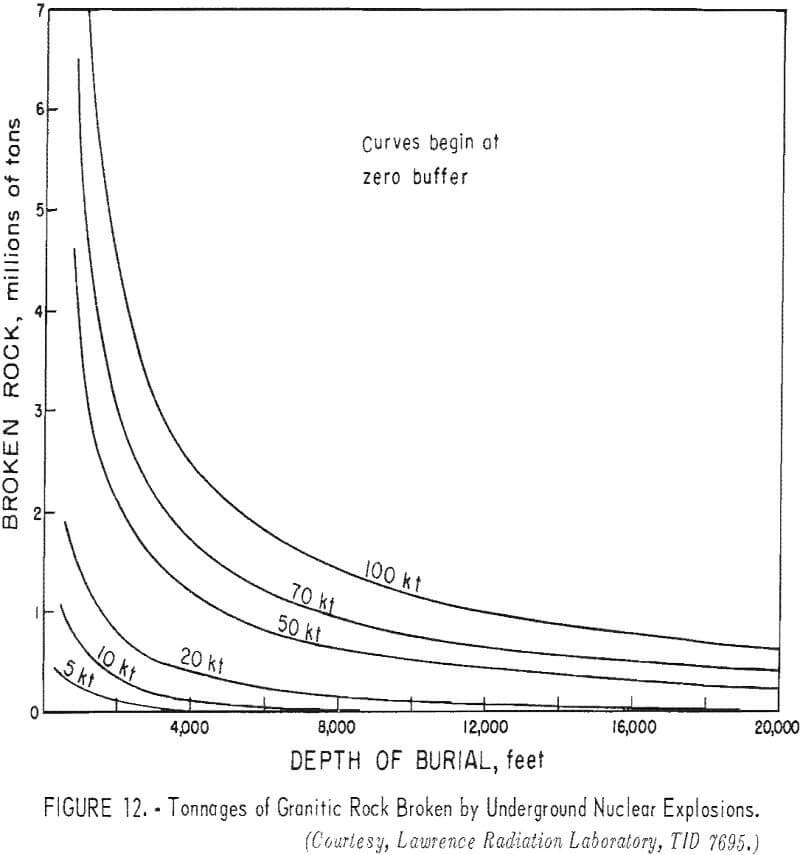
Curves have been developed to show the cavity radius as a function of the depth of burial (fig. 10), the chimney height as a function of the depth of burial (fig. 11); and the tonnages of rock broken as a function of the depth of burial (fig. 12) for different yields.
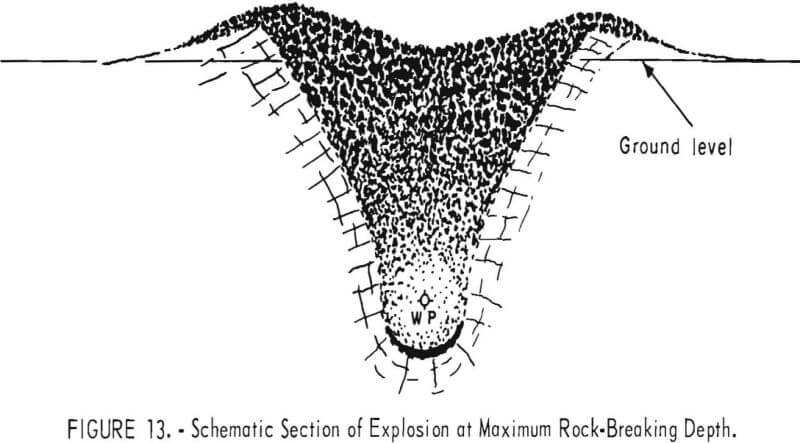
An example of an emplacement for maximum rock breakage was the Sulky nuclear explosion in hard, dry basalt. The explosion, which had a yield of approximately 87 tons of TNT, took place on December 8, 1964. Fired at a depth of 90 feet the explosion formed a mound of rubble with a maximum height of 25 feet above preshot ground surface and a diameter of over 160 feet. Scaled depth of burst was 184 feet/kton. Radiation levels were
sufficiently low that personnel were able to move freely about the rubble mound within a few days and excavation of trenches and broken rock began within a month. A schematic cross section of a shot designed for maximum rock breakage is shown in figure 13. Fractured rock from a cratering nuclear explosion in hard basalt is shown in figure 14.
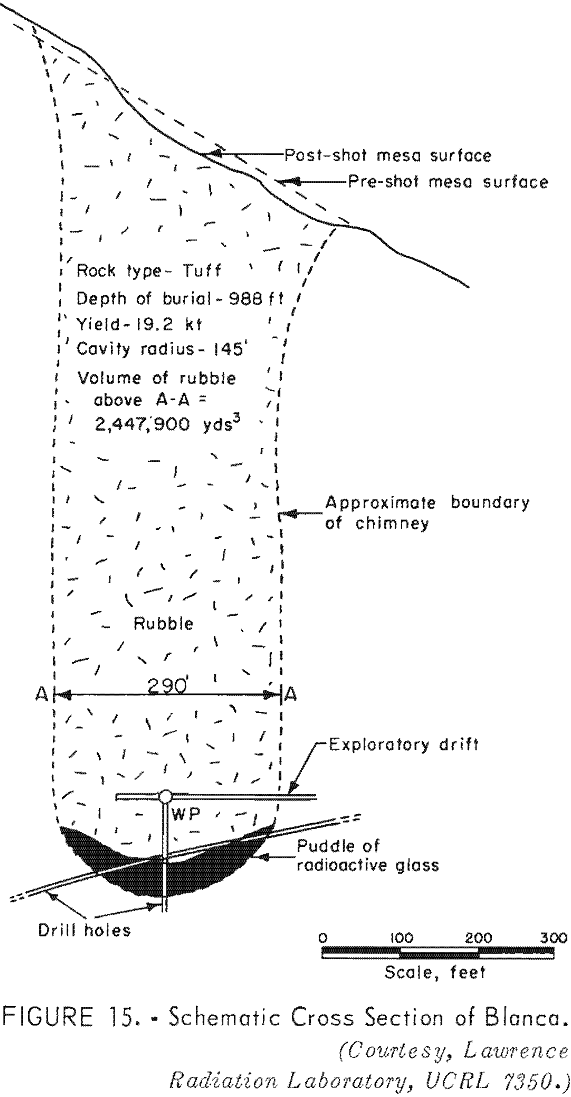
The Blanca explosion was a 19-kton explosion fired in a tunnel in bedded tuff on the edge of a mesa. The bulk density of the bedded tuff in situ was about 1.9 g/cm³. The explosion was at a vertical depth of 988 feet, and the nearest point on the surface was 835 feet. A dome section of the mesa 1,600 feet in diameter was moved upward about 25 feet at the center then settled back. About 15 seconds after the explosion the cavity collapsed to the surface (fig. 15). The rubble zone, an inverted cone with a radius of 500 feet and a height of 1,100 feet, was estimated to contain 15 million cubic yards or 22 million tons of rock. About 0.05 percent of the total radioactivity was released to the surface.
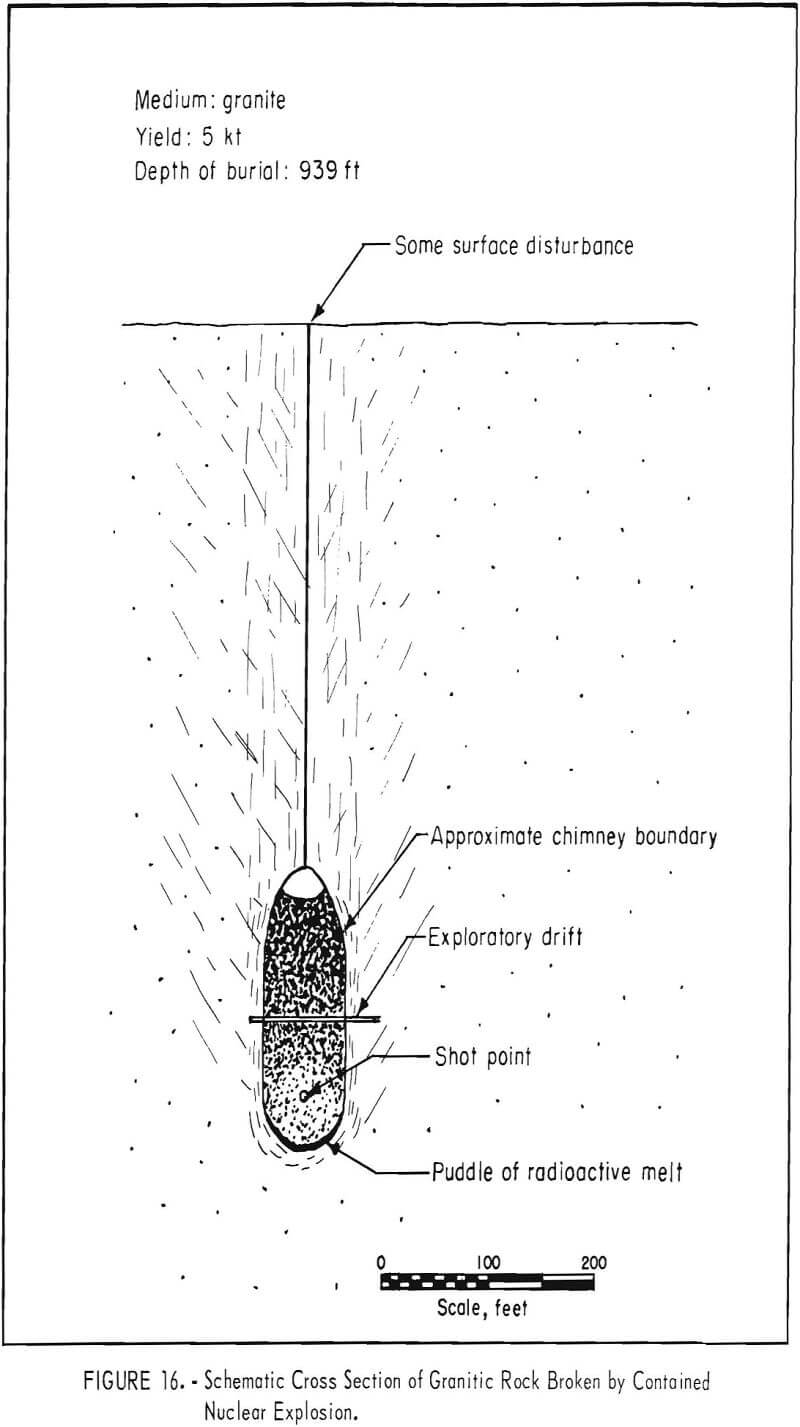
The Hardhat test, a contained explosion, was a 5.4-kton shot fired from a 36-inch diameter drill hole in granite (specific gravity 2.67) at a depth of 939 feet on February 15, 1962 (fig. 16). The surface raised 4 feet and sank back; permanent displacement was about 2 feet. A cavity 126 feet in diameter was formed. Eleven hours after the explosion the roof began to cave, eventually forming a chimney that reached a height of about 300 feet and contained an estimated 220,000 tons of broken rock. A drift was driven through the caved chimney 90 feet above the point of detonation, and 3,000 tons of broken rock was drawn from two 4-foot finger raises. Material mined appeared well broken; the size distribution appeared to be suitable for extraction by methods similar to caving or in-situ leaching (fig. 17), After the cavity collapsed, small amounts of radioactive gases were detected on the surface.
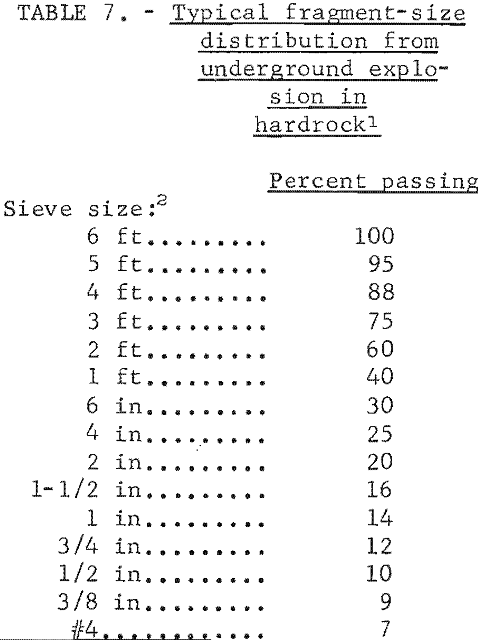
Porosity of the broken material after a nuclear explosion ranged from 18 to 35 percent in tuff. The porosity of the Hardhat chimney in grandodiorite was about 28 percent. Factors that influence the overall rubble size distribution and maximum rock fragment size and shape included the following: (1) characteristics and frequency of natural fractures; (2) physical properties of the in-situ rock; (3) explosion yield; and (4) depth of burial. Typical fragment sizes are summarized in table 7.

Public Liability
In any undertaking by industry to apply nuclear explosives, industry is taking the assets of a going business and investing them in a new idea. Accordingly, it must assure its stockholders that those assets will not be in danger. In order to provide assurance to industry and to the public, the Atomic Energy Act of 1954, as amended, authorizes the Atomic Energy Commission to execute indemnification agreements with its contractors who are engaged in activities for the benefit of the United States involving a risk of public liability for a substantial nuclear incident. These agreements indemnify against claims for public liability for nuclear incidents arising out of or in

connection with the contractual activity in the amount of $500 million over and above any financial protection (insurance etc.) that the Commission may require of its contractors. The act also provides that in no event shall the aggregate liability for a single nuclear incident for persons indemnified exceed this amount. Thus far, the Commission has not required its contractors to procure such financial protection.
Under existing statutory authority, fabrication, emplacement and detonation of the nuclear explosive in any such application by industry must be done through the AEC. Those of its contractors who provide this service for the AEC have been extended indemnification agreements. Such agreements not only indemnify the contractor but any other person who may be liable for public liability. Accordingly, anyone who participates with the AEC in an experimental application of nuclear energy, such as fracturing copper deposits, will have assurance because of the existence of such an agreement that all risks for public liability will be covered.
Safety Problems
The use of explosives is an important part of all mining operations. The handling of the explosives must be accomplished safely and the effects anticipated and controlled. The problem of what effects the air and ground vibrations resulting from blasting have on structures of various types has existed from the time explosives were discovered and developed for mining purposes. Nuclear explosives, because of the large amount of energy involved, amplify the safety problems connected with air blast and ground shock and introduce a new problem, radioactivity.
Because the general public is directly involved in the blasting vibration problem, many investigations have been conducted. The behavior pattern for the effects of vibration from quarry and mine blasting can be predicted. Tests and follow-up investigations have provided a basis for reliable preshot assessments of potential hazards from the effects of nuclear explosives.
Air Blast
Air blast from a surface explosion results from the rapid expansion of the hot gases. The explosion causes a shock wave to form in the air and move outward at high velocity. Waves move radially along the surface of the ground or may rise and be ducted and refracted by atmospheric conditions.
The air-blast wave from a buried explosion may consist of two pulses, one that results when ground shock reaches the surface and one that results when gases are vented. Both pulses decrease with charge burial. The pulse caused by venting gases becomes zero when the explosion is completely contained. Where nuclear charges have been fired in basalt and rhyolite rock, peaks from venting gases have been less than those that were ground-shock induced. The zone of air-blast damage for nuclear explosions at depths contemplated for fracturing copper deposits can be expected to be well inside the region of ground-shock damage.
Ground Shock
Ground shock or seismic effects of a nuclear explosion are similar to those of conventional explosives. Ground shock results when the shock wave caused by an explosion is transmitted outward through the rock. Criteria for estimating damage to residences and engineering structures has been established. Particle velocity or acceleration in terms of gravity are used to measure particle motion.
Damage appears as cracks and as structural deformation. A recent statistical analysis by the Bureau of Mines suggested that a particle velocity of 2 inches per second is a reasonable point of demarcation between shock levels in the safe zone and the damage zone.
Ground motion resulting from nuclear explosions and the effects on buildings and structures has been studied. A surface velocity of 10 cm/sec was determined as a damage threshold. Damage to engineering structures begins at a much higher velocity, depending on the type of structure. For many structures it may be about 100 cm/sec. A formula was presented by Hansen and Lombard to calculate the peak velocity at a distance from the explosion.
The peak vertical component can be approximated at any distance from the explosion by the formula:
V = K W2/3/R3/2 cm/sec
Where K = 1.4 x 10-² for explosions in alluvium,
= 3.5 x 10-² for explosions in tuff,
= 8.6 x 10-² for explosion in granite,
R is the range in kilometers,
and W is the energy yield in tons.
A special consideration is the shock damage to mine workings. Shock damage to unsupported mine workings in granitic rock has been plotted as a function of explosive yield and range by Hansen and Lombard (fig. 18).
Minor damage includes limited spalling, minor offsets of shaft guides and track and slight damage to mounted equipment. Complete closure is defined as complete collapse of the mine opening from top, sides, and bottom. Although probably not important in a mining operation involving a single nuclear explosion, this phenomena may introduce a serious problem at operations where several nuclear explosions separated by periods of ore extraction are involved.
Radioactivity
Radioactivity that is vented to the atmosphere at explosion returns as fallout. For purposes of fracturing and leaching a copper deposit in situ, the nuclear explosive would be placed at a depth such that the radioactive products are completely contained or, at most, only an insignificant fraction escapes. When the explosion is completely contained radiological safety problems encountered from activity will be introduced in the ore from the following sources :

- Radionuclides that result from fission or fusion products of the explosion.
- Radionuclides that result from the activation by bombardment of the ore and materials in the nuclear explosive with neutrons released in the explosion neutron activation products).
Eliminating the possibility of hazards from fallout) potential radiation hazards associated with fracturing a copper deposit with a nuclear explosive and subsequent in-situ leaching to recover the copper are: (1) Radiation hazards to off-site environment) (2) radiation hazards to on-site personnel, and (3) contamination of the copper product.
Radiation Hazards to Off-Site Environment
Assuming that the nuclear explosion was contained and there was no significant venting of the radioactivity to the air, the off-site environment may still be subjected to radionuclides from waste products or from contamination of ground water. Some activity, particularly tritium, may be carried into the ground water flow. Ordinarily, ground water moves slowly, requiring years to travel a distance of one mile. Underground disposal of radioactive wastes has been practiced for many years. The distribution in ground water of radionuclides has been studied with the conclusion that the ground water transport of radionuclides, following the initial explosion-produced distribution may not create any unmanageable problems. The radiation hazards to off-site environment should be controllable, but a study of ground water movement and waste-solution disposal will be advisable.
Radiation Hazards to On-Site Personnel
Radiation hazards to on-site personnel can come from two sources: radio-nuclides may build up in circulating solutions from an in-situ leaching operation; they may be present in shattered ground through which development openings are necessary.
The Oak Ridge National Laboratory is studying potential safety problems that may result from radioactive contaminants in the processing cycle and the copper product. Copper ore samples from New Mexico and Arizona have been mixed with radioactive debris from nuclear explosions on the Nevada Test Site and bombarded with neutrons by equipment at Oak Ridge National Laboratory. Preliminary studies indicate that expected concentrations of radioactivity in process solutions and product will be manageable. Planning may be required in designing the operation particularly with respect to disposal of waste solutions.
During a mining experiment on the Nevada Test Site one year after the explosion, the average exposure was 11 millirem per working shift corresponding to 2.2 rem per year. The National Council on Radiation Protection (NCRP) recommended maximum permissible whole body dose for workers is 3 rem per quarter year or 5 rem average per year beyond age 18.
Contamination of Copper
In tests by Oak Ridge National Laboratory ruthenium was the only fission product which significantly followed the cement copper. The chemistry of ruthenium is very complex and the results obtained with ruthenium traces may or may not be representative of those that would be obtained in actual practice. The indicated amount of damaging radioactivity that will follow the copper is not great and does not appear to present serious problems. Conclusive evaluation of potential separation methods cannot be determined until actual process products are available. Ruthenium would probably be eliminated from a copper product when it was removed from solution or refined by the electrolytic method. Methods of removing ruthenium from solution are known.
Application of In-Situ Leaching to Nuclear Fractured Deposit
Copper deposits differ greatly, and no single method is applicable to all deposits. Regardless of method or preparation, successful application of a leaching method to recover copper from ore in situ requires existence of the following favorable factors in high degree:
- Non-acid-consuming host rock.
- Host rock that will not decrepitate to seal intrarock cavities and block solution flow.
- Rock sufficiently fractured to permit access of solution to copper minerals.
- Copper minerals that dissolve within required time limit.
- Copper minerals concentrated largely along fracture planes of rock.
- Sufficient pyrite in the deposit to form sulfuric acid and ferric sulfate.
- A solid impervious surface under or surrounding the deposit.
- Ability to recirculate the solution through the ore many times without excessive loss or contamination.
- Availability of adequate water at low cost.
In addition to the limitations inherent to in-situ leaching, some further factors arising from the characteristics of nuclear explosions become important. Some important factors requiring investigation are:
- Hydrology pattern including ground and surface water movement.
- Safety considerations in connection with proximity to engineering structures that may be affected.
- The effect of radionuclides on plant design.
- The effect of radiation on bacteria.

- Area available for waste solution disposal.
- Population density and public attitude.
Copper deposits that may be fractured with a nuclear explosive and leached in situ are divided into two types: Those that can be fractured with a single explosion, and those that require more than one explosion.
Either intermediate or contained placement of a nuclear explosive may be selected to fracture a copper deposit for in-situ leaching; the choice will be controlled by the position of the deposit and adjacent development. Once broken, the optimum method of percolating the solution through the ore may be either flood or open drainage depending on the condition of the surrounding rock the texture of the broken material, and the method of solution recovery.
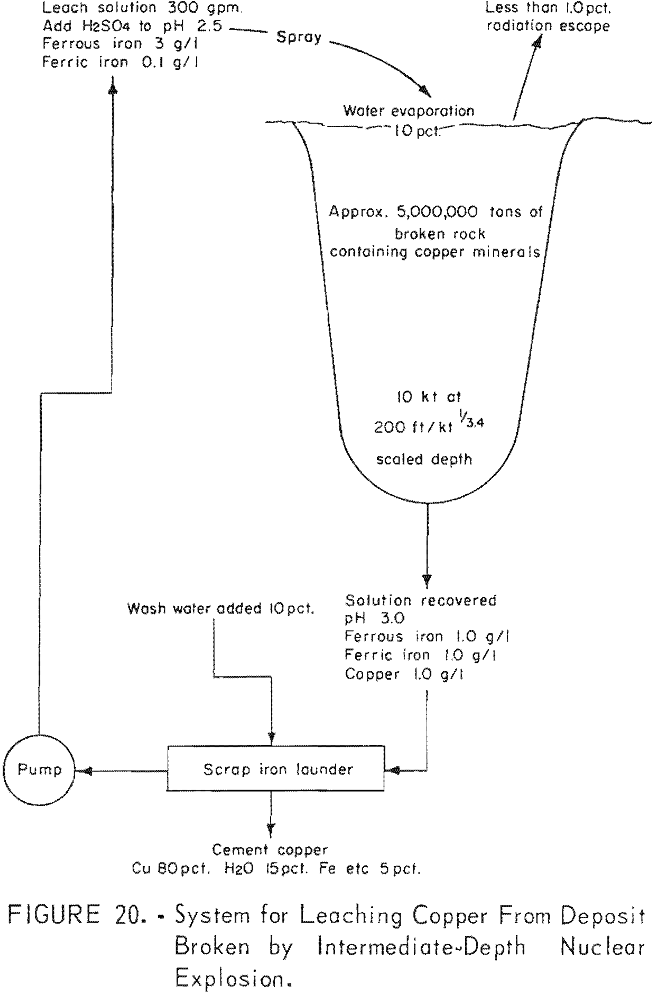
An intermediate explosion to fracture the surface but not close enough to crater or release a significant amount of radioactivity to the atmosphere may be the most efficient, if there is not too much overburden for two reasons. First more rock is broken per energy unit by the explosive. Second, solutions can be more easily and economically distributed on the exposed surface and recovered from the near surface sump (fig. 19). A diagrammatic system for leaching copper from ground broken in this manner is illustrated in figure 20.
Solution would be distributed by plastic pipe on the surface. Where the rock was impervious and solution was contained; it could be recovered by pump from the bottom of the crater. A characteristic of the nuclear explosive is that the rock immediately below the bottom half of the cavity is compacted. In some formations; this compaction may form a depression sufficiently impervious to contain the solutions from open-drainage application they could be recovered by pumps in drill holes. However, in some cases where the rock is not impervious, it may be necessary to develop drifts and crosscuts to collect the solution either from an adit or a shaft.
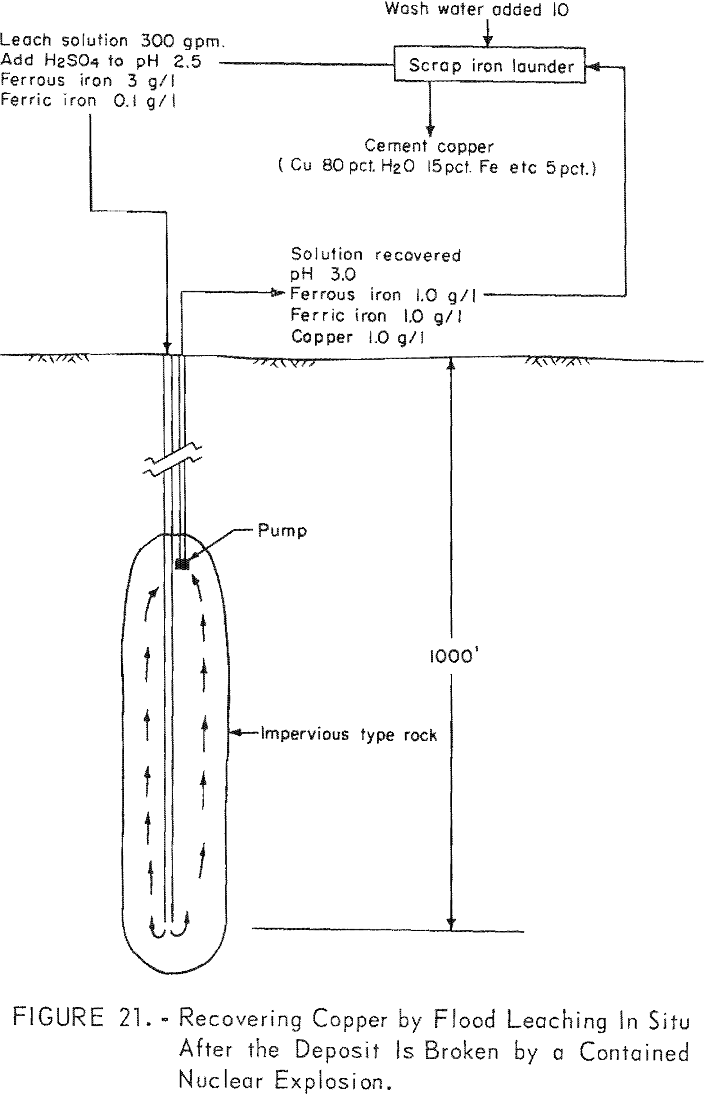
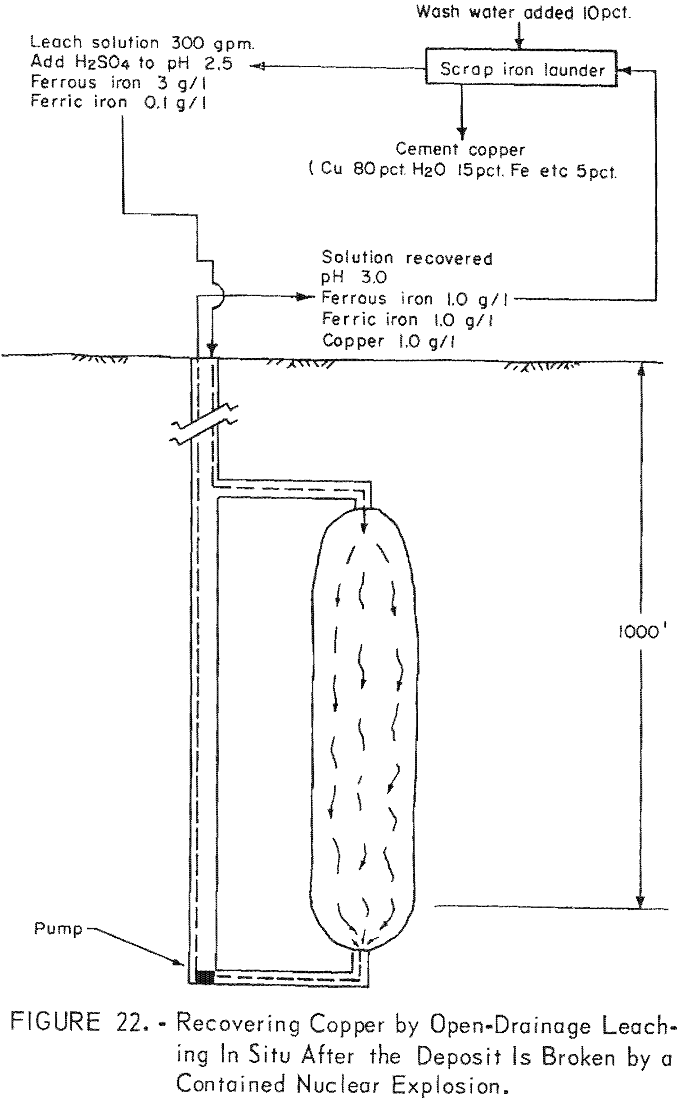
Many deposits containing copper in leachable minerals are formed near an erosional surface. However, the deposit may have been formed in a past geological period and may now be covered deeply by more recent rock. These deposits can be economically broken only by a contained nuclear explosion. After the deposit is fractured, the solution could be percolated through the rock either by the flood method as illustrated in figure 21, or by the open-drainage method as illustrated in figure 22.
The development for applying solutions by the flood method may be most economical as two drill holes, one to the top and one to the bottom of the broken ore would be sufficient to apply and withdraw the leaching solution. Obviously, this method could be used only where the surrounding rock was impervious to the extent that the solution was contained and no significant loss occurred.
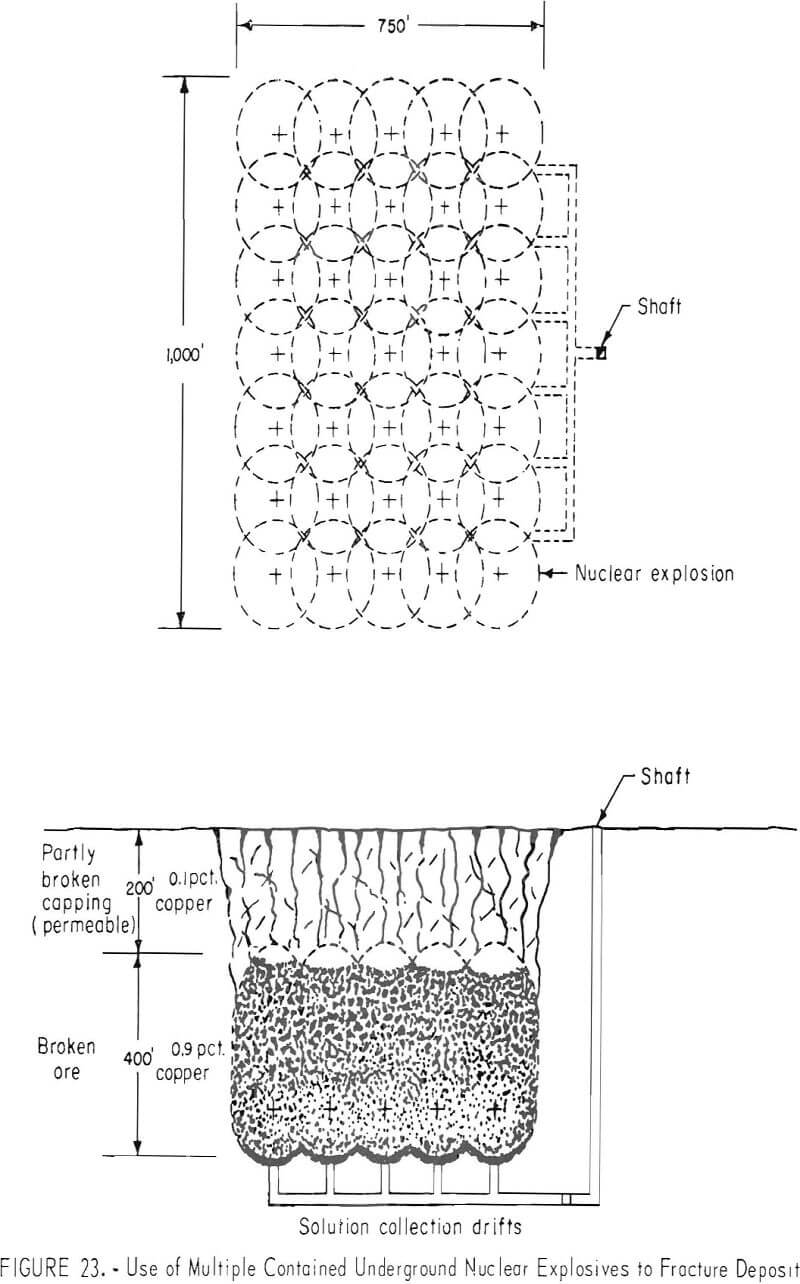
Contained nuclear explosions may be limited in size by safety considerations to less than 100 kton in most locations and to less than 10 kton in many locations. This restriction may not be serious because the conformation of many deposits is such that they can be more efficiently fractured by multiple explosions. This may not be the most economical method of breaking rock but, overall, may be the most economical method of fracturing and preparing ore for in-situ leaching (fig. 23).
Economics of Nuclear Fracturing and In-Situ Copper Leaching
In-situ leaching operations have not been charged with the cost of breaking and fracturing the deposit in the past. This cost has been carried by the primary mining operation that required the development. This cost is included
in the following estimate.
The cost per ton of breaking rock with nuclear explosives is shown in table 5. These costs are for explosives placed for maximum rock breakage in the form of a retarc. The ratio of rock broken in a retarc exceeds that of a contained explosion by a factor of 6 to 1 at the minimum contained depth. This ratio increases with the depth of placement beyond the minimum contained depth. Fracturing a mineral deposit may be expected to require deeper placement than this optimum depth and is estimated to break about a sixth the indicated tonnage of table 5 or to break rock at six times the cost per ton. The cost per pound of recoverable copper for breaking and fracturing a deposit is estimated in table 8.
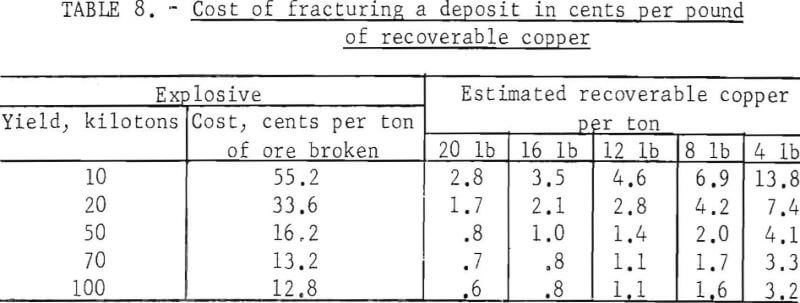
The plant and equipment necessary to recover copper from leach solution by metallic iron is simple and inexpensive with the exception of pumps and pipe required to circulate the solution; these require costly corrosion-resistant materials. The cost of plant construction varies with many factors Estimated cost for typical plants is shown in table 9.
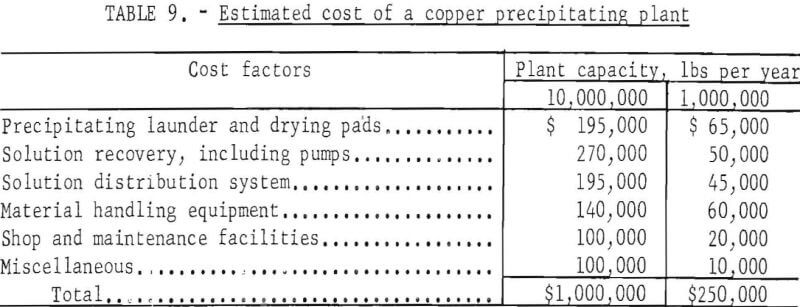
Assuming a 10-year operation, the capital investment per pound of copper produced is 1 cent for the larger plant and 2.5 cents for the smaller plant.
A plant producing 10 million pounds of copper per year is estimated to require about 25 employees, while one producing 1 million pounds of copper per year might require 6 employees. It is estimated each employee will cost $6,511 per year.
Operating supplies include sulfuric acid, scrap iron, power, and miscellaneous items. Estimated operating costs for the two plants are shown in table 10.
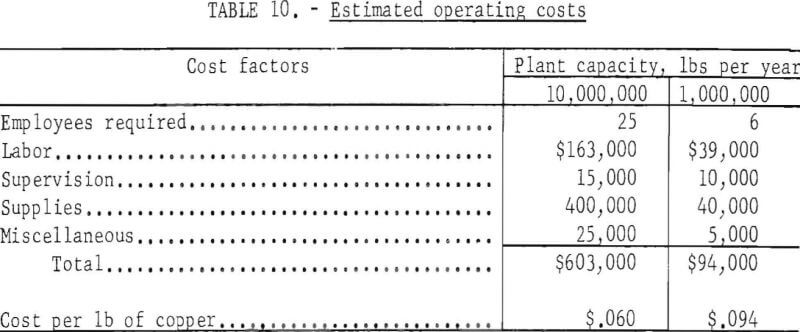
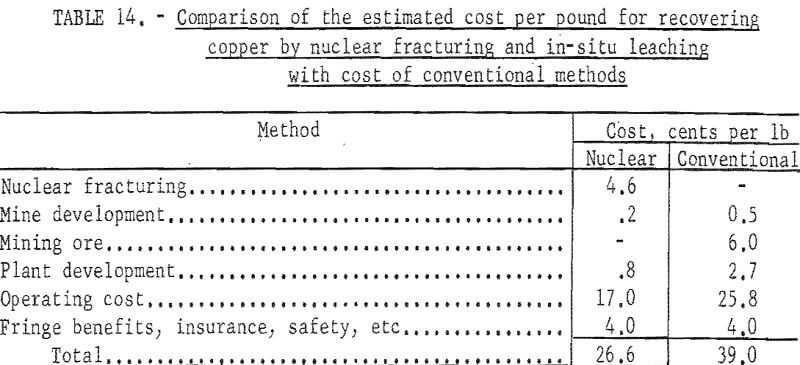
Assuming repair and replacement are equal to capital cost, and allowing for general expenses, fringe benefits, insurance, safety, and refining and marketing, the total cost of producing copper by in-situ leaching (not including the cost of breaking and fracturing the ground) is estimated as shown in table 11.
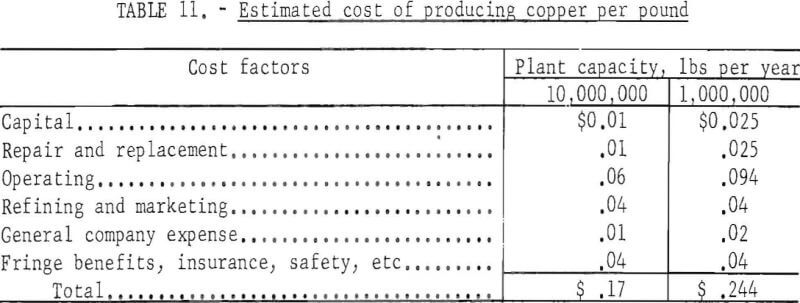
General company expense is estimated to be somewhat lower for the larger plant. To these totals must be added the cost of breaking and fracturing the deposit which, as indicated in table 8 may range from 0.8 to 13.8 cents per pound of copper.
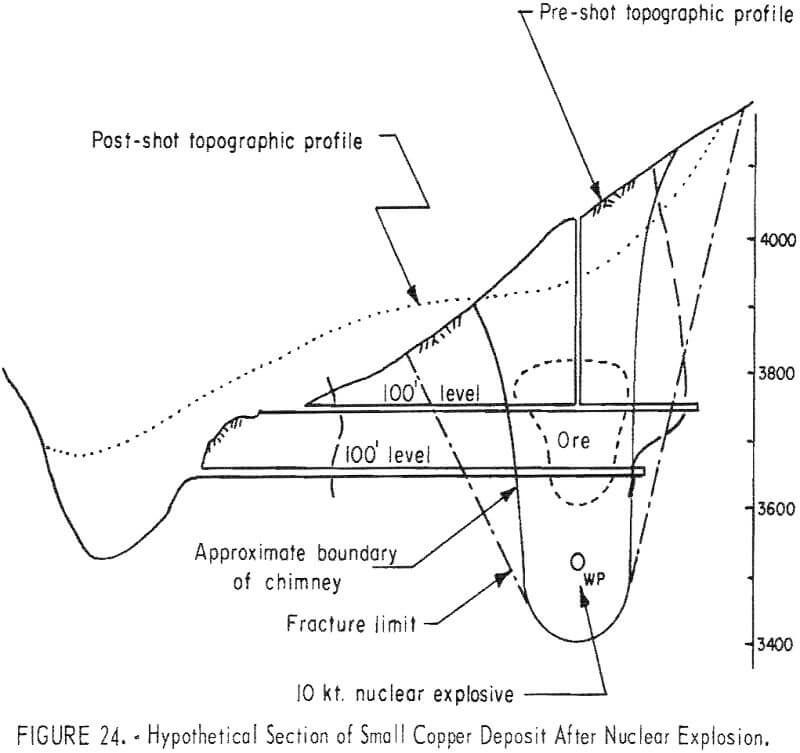
A typical small deposit is shown on figure 24. The outlined ore body is assumed to contain 500,000 tons with a grade of 2.5 percent copper in the minerals chalcocite, bornite, chalcopyrite. The rock immediately above the ore body is assumed to contain 1.5 percent copper mostly in the silicate and carbonate minerals, and the 100 feet near the surface is assumed to be leached capping with a copper content of 0.15 percent. The copper content of a column 200 feet in diameter and 400 feet high through the deposit is shown in table 12.
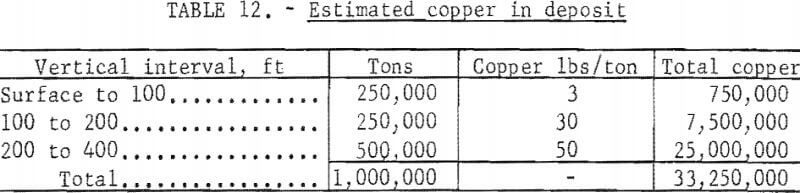
The leached capping of this deposit contains 3 pounds of copper per ton; the sulfide ore contains 50 pounds. Assuming that the leached capping initially contained 50 pounds of copper 47 pounds or 96 percent has been leached and removed. Not all of this copper will be leached during the life of leaching operations but the assumption that 70 percent can be leached is reasonable.
The deposit is contained in a column of rock 400 feet high and 200 feet in diameter. A nuclear fracturing explosion with the broken rock reaching the surface (fig. 13) is indicated for efficient leaching.
Referring to figure 9, a 10-kton explosive at a depth of 500 feet is indicated for a cavity radius of 100 feet. The estimated chimney height (fig. 10) for a shot of this size is about 400 feet and the chimney might be expected to reach the surface from a depth of 500 feet. The indicated tonnage broken (fig. 11) is 1.0 million tons.
The cost of a 10-kton explosive from table 5 is $550,000 to which must be added the cost of the safety study, which can be determined only after site has been selected. For this study, the safety investigation is estimated to equal the cost of the explosive making a total of $1,100,000 for breaking ground. Assuming 70 percent of 33,250,000 pounds of copper or 23,275,000 pounds will be recovered, the cost per pound of copper for breaking the deposit is 4.8 cents.
An annual production of 2.3 million pounds of copper is indicated. Referring to table 5 the costs of an operation are estimated to be somewhat less but approach those for the smaller plant and the total cost of producing copper not including royalty, taxes and profit are estimated in table 13.

To illustrate the possible use of multiple explosives, a deposit of copper ore 750 feet wide, 1,000 feet long, and 400 feet thick is assumed. A leached capping 200 feet thick and containing 0.1 percent copper is assumed to cover the ore which contains 1.0 percent copper in the leachable mineral chalcocite.
The copper content of the leached capping is 10 percent of that in the ore and indicates that 90 percent of the copper in the original material has been leached. Eighty percent of the copper is assumed to be recoverable in a period of 10 years. Eighty percent of the copper is also assumed to be recoverable by flotation.
The deposit is estimated to contain 24 million tons of copper ore that contains 384 million pounds of recoverable copper by either method. Multiple explosions are required to fracture the deposit.
Production costs by the nuclear fracturing in-situ leaching method includes those for fracturing the ore with 35 nuclear explosives in drill holes, preparing a 700-foot shaft and 17,000 feet of drifts and raises to collect the solution constructing a copper precipitating plant, and operating for 10 years.
Production costs by conventional methods include those for development of a caving mine, development of a 6,700-ton per day concentrating plant, and operation for 10 years. The estimated costs of recovering copper by the two methods are compared in table 14.