Table of Contents
Before the event of ore dressing, crude ores were shipped directly to the smelters, or the refineries, with the shipper paying the freight and treatment charges. These charges varied with the type of ore and the distance from the smelter. In all cases they were many times our modem milling costs. While the smelter recovered the principal values, substantial losses of other metals and even penalties, such as for zinc at a lead smelter were common. With the high cost of the smelting operation only high grade ores could be shipped and treated at a profit.
The day of high grade ore deposits is practically over. The tremendous production caused by wars and intense industrial development has demanded so much metal and mineral production that only medium and low-grade ore deposits are left available for development. Development at depth of complex mineral ore bodies is now being undertaken and these ores are being treated by methods which were unknown even a few years ago.
An example of this is in the use of the “sink- float” process which has been recently introduced. In this process certain minerals can be separated from a large portion of gangue, or waste material. A preliminary crushing to the necessary fineness is made, in some cases as coarse as 2″, and then the ore is subjected to a separation through a heavy mineral suspension. The concentrated values are then further treated by finer grinding, flotation, or whatever indicated treatment is required to make a finished product. In other words, the “sink-float” process, or preliminary concentration by gravity, is now being employed to make available an enriched mill feed in many instances where the original ore is complex, or of relatively low grade.
The scarcity of undeveloped high grade deposits has brought about the more liberal employment of milling and concentrating plants at the mines, or of custom milk to serve definite mining areas. These, in many instances, enable small producers, and low grade properties, to produce at a profit. By disposing of their output to the custom mills the miners can often make a reasonable profit and the owners of the custom mills are able to ship their concentrates to the various smelters profitably.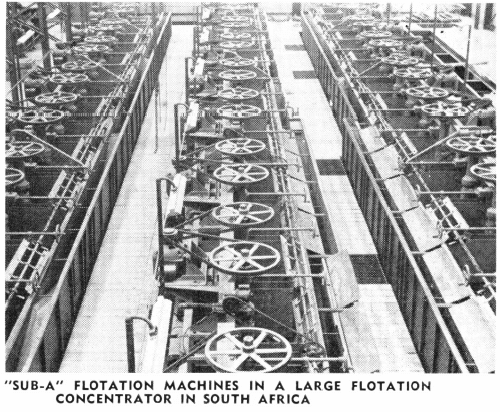
There was a time when it was not considered essential nor profitable to separate the copper from the lead in lead concentrates. But today, owing to the demand for copper, and also due to the refinements brought about in selective flotation, a great many producers are making this profitable separation with the respective concentrates going to their proper smelters.
This same trend is true with gold which, some years ago usually accompanied the copper-lead concentrates to the smelter. Today, owing to recent developments in metallurgical equipment, a large portion of the gold content can be recovered through the use of jigging, table concentration, or selective flotation. In many cases gold and silver bullion can be produced at the concentrator. This represents mostly clear profit, for this bullion can be shipped direct to the mint.
Owing to the present situation, where more of the low grade gold deposits will come under observation and where areas containing scattered gold values will be resurveyed, the need is obvious for small semi-portable gold milling units. These may include standard equipment for amalgamation, flotation or cyanidation or a suitable combination of these treatment methods. These may be so constructed that they will handle 10-25 tons per day and will, in addition to accurately sampling the deposits, also serve to produce a sizeable revenue. Through the use of small mills of this nature the proper treatment methods for a larger, more permanent mill installation may be worked out. The elements of risk, both of ore grade and method of treatment, is minimized by these portable mills. This work necessarily will be carried out under the supervision of competent metallurgical personnel, and should first be checked by batch laboratory tests to secure the full benefit of this preliminary work.
Here a resume is given of various phases of milling to show that the progress has been gradual and that the same fundamental principles which guided us in the past are still important principles to recognize in modern milling practices.
The object of modern milling is to reject the barren and worthless gangue or rock material and to concentrate in the least bulk the valuable mineral, or minerals, for shipment to their respective markets. In treating complex ores this means making more than one concentrate in order that—for example—the lead concentrate may go to the lead smelter, the copper concentrates to the copper smelter, and the zinc concentrate to the zinc refinery. If possible, any gold that is present in the ore may be recovered at the concentrator in the form of bullion, for shipment direct to the Mint.
Milling History Amalgamation
Although most of the processes included in the modern mill have been developed within the last fifty years, amalgamation of gold and silver was known and used hundreds of years ago.
The use of amalgamation for the treatment of gold ores, especially for the small mill, is still an important part of modern milling. Plate amalgamation, which consists of grinding the ore and then passing this ground ore pulp over a large surface of mercury-coated plates, is still in use. However, it is rapidly being replaced by the amalgamation in a clean-up barrel or pan, of a small portion of the original feed in which the gold values have been concentrated. This concentrate containing the gold minerals is recovered by the modern Mineral Jig, and/or the “Sub-A” Unit Flotation Cell. Continuous amalgamation in a Wheeler type amalgamator or in an amalgam barrel has possibilities as soon as the feeding and use of mercury is thoroughly studied.
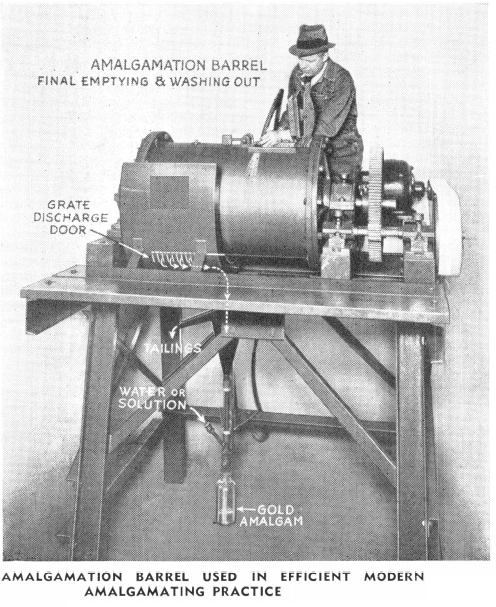
Although the process of amalgamation is one of the oldest used in the history of milling, it has been sadly neglected in milling practice in recent years. Often this has been due to the proximity of the mine or mill to the smelter. Thus, the relative economies of treatment at the mine, or direct shipment to the smelter, have not been given sufficient study. The concentration of gold and/or silver values at the source, utilizing modern amalgamation methods, or a combination of roasting and amalgamation, may be more advantageous to resort to than a combination of amalgamation and cyanidation. In certain mining sections there will undoubtedly be an increase in the number of custom mills. At these mills high grade silver-gold concentrates can be marketed and they will then recover the precious metal content by any number of suitable flowsheet arrangements. Certain ores are amenable to treatment in amalgamation barrels, or tube mills adapted to operate as amalgam barrels, and may be handled either on a batch or continuous basis.
Electric amalgamation and special mercury compounds have been tried but the fouling of the mercury is the main drawback to their practical use.
Concentration of Minerals
Mechanical concentration is based on the difference in specific gravity of the minerals or materials to be separated. The crude application of the process, such as the use of hand concentrating tubs and pans, riffle boxes, blankets, and goat or sheep skins (Golden Fleece) dates back to ancient times, and was used mainly for recovery of gold and silver. Later, however, it was also used for tin, lead and other metallic ores. These crude devices were followed by the buddle, the Gilpin County Bumper, the Wilfley Table, and Harz Type Jig.
The Wilfley Table, invented in Colorado in 1895, together with the development of the mechanical jig, brought mechanical concentration to a fine degree of perfection on all materials, except slimes. It remained for the flotation process, some 20 years later, to successfully treat this slime material. During this interval many machines for treating slimes were tried and used, such as the Frue Vanner and slime tables, but these were all replaced by the flotation process.
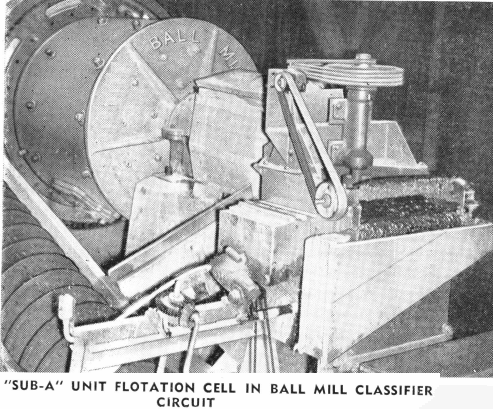
During the last ten years more and more recognition has been given to the advisability of recovering heavy metal values at as coarse a mesh as possible. This has been accomplished through the use of a jig and/or a unit flotation cell in the ball mill-classifier circuit. This practice—of recovering mineral at as coarse a mesh and as soon as possible, has long been followed, and is still as sound a principle as ever.
On slime values, particularly where oxides are treated, such as gold, tungsten or tin, the automatic Buckman Tilting Concentrator has proved to be a big factor as a cheap and efficient means of recovering fine mineral values. In Africa a rotating drum type concentrator has been used successfully in performing this same function.
Concentration of gold bearing sulphides by flotation or jigging, followed by intense grinding and cyanidation of these, has been a noticeable feature of many new plants in Canada. This flowsheet permits a coarser grind of the main bulk of the tonnage with an attendant increased recovery and reduction in overall costs.
Bulk Flotation
The need for the flotation process was very urgent, as millions of dollars in values were being lost every year in unrecovered slime in the tailings. Under these conditions scientific investigators and engineers were encouraged to find some process to eliminate this loss. About ten years of trial and mostly failure passed before the froth flotation process, as known today, was developed.
This process is based on one main fact, that a sulphide mineral particle and also other minerals properly treated, will adhere to the surface of an air bubble, and that gangue and rock particles will not adhere to the bubble. With this fact in mind, it is easy to see that here is a process which will separate sulphide mineral particles irrespective of specific gravity, and also of size, within certain wide limits. This is termed bulk flotation, which is the separation of all the sulphides or minerals from the gangue material.
The application of this process to mill operation, although very simple today when proper reagents and equipment are used, is affected by many chemical and physical conditions. These had to be investigated and necessary means found for their control.
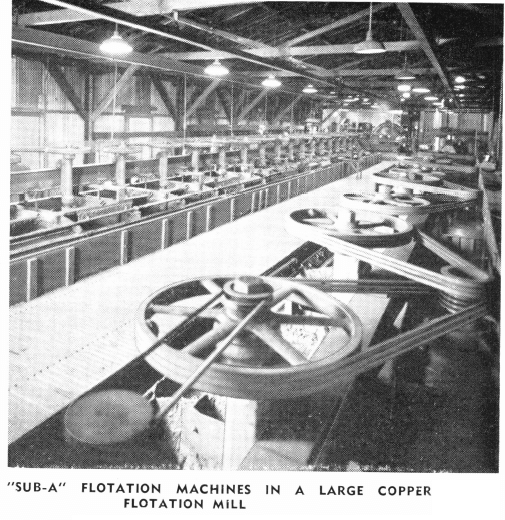
Since the adherence to the bubble is a surface phenomena, any change in the surface properties of either the mineral particle or the bubble will affect the floatability. For the best results in bulk flotation, all additions of reagents or conditions of treatment must either increase or retard this float-ability of the sulphide particles in respect to gangue particles. Because the sulphide slime particles are very readily acted on by oxygen and other chemically active constituents of the pulp, thereby reducing their floatability, the best results are obtained by removing the sulphide particles as coarse as possible—without unnecessary sliming and subsequent loss in the tailing. This is best accomplished by a “Sub-A” Unit Flotation Cell placed in the ball mill-classifier circuit to remove the coarse sulphide particles before they are returned to the grinding mill where they would be unnecessarily slimed. The product obtained from the unit cell is usually a high grade concentrate.
Selective Flotation
Bulk flotation solved the problem of the loss of slime values in the tailing of the gravity concentration plant but still did not solve the problem of the separation of the different sulphides from each other. In many complex ores the iron sulphide contained no precious metals and was of no commercial value as pyrite. In this case it would be very desirable to leave this pyrite in the tailing. This would save the handling, freight and treatment charges of this added bulk in the valuable gold, silver, copper, lead, zinc or other concentrate. With most ores containing lead and zinc sulphides, the production of a separate lead concentrate for shipment to the lead smelter, and of a separate zinc concentrate to ship to the zinc smelter, makes for a profitable operation. It only gravity concentration or bulk flotation were used the ore would probably prove unprofitable to treat. In the latter case, if a bulk concentrate were made containing the lead and the zinc, the zinc not only dilutes the concentrate on which more freight and treatment charges have to be paid, but, at the lead smelter, the zinc content or a portion thereof would be penalized. This, in most cases, is an avoidable loss, since it would be paid for if shipped as zinc concentrate to the zinc smelter.
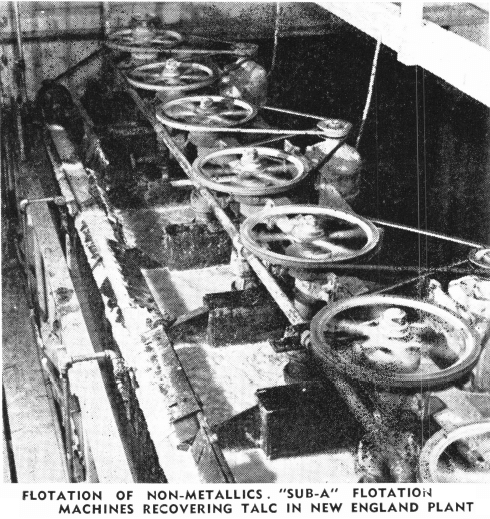
The separation of copper and iron sulphides, the specific gravities of which are fairly close together, was almost impossible with gravity concentration. However, with lead and zinc sulphides, the specific gravities of which are quite different, gravity concentration gave excellent results on jig sizes, with only fair results on closely sized table feeds, and on slimes the separation was impossible. Since most of the complex ores are intimately mixed and require some degree of fine grinding, selective flotation is necessary for the successful treatment of most of the present day ores.
Flotation of Nonmetallic Minerals
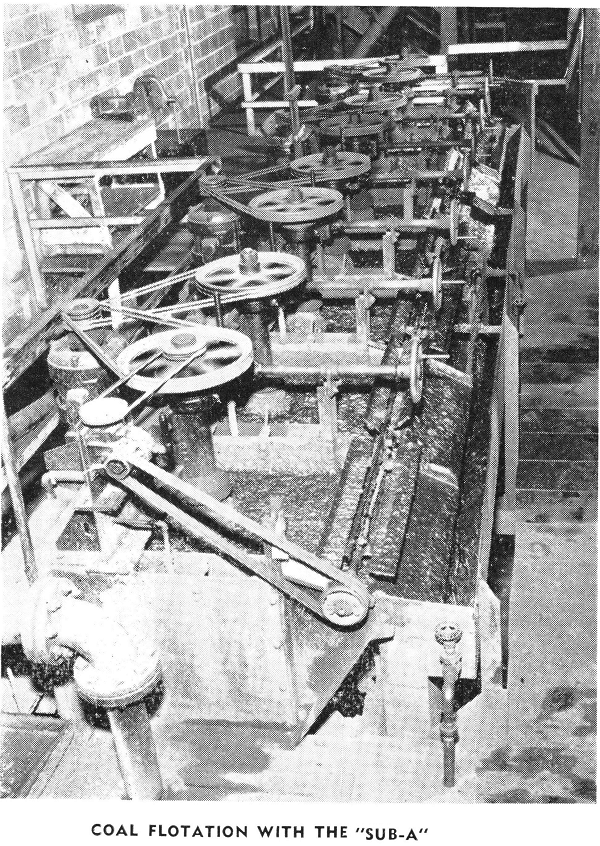
One of the widest fields in flotation is its application to treatment of non-metallics. Rapid strides have been made in this field in recent years, due in a large part to development of new reagents.
Many non-metallics are now being successfully beneficiated, such as potash, phosphates, fluorspar, barite, glass sand, coal, and many others.
Flotation has been applied to many problems in industry other than the recovery of material from crude ores. For instance, ink and other extraneous matter is being removed from repulped used paper. Another example is peeling of grain to remove hulls and other impurities, using modified flotation equipment.
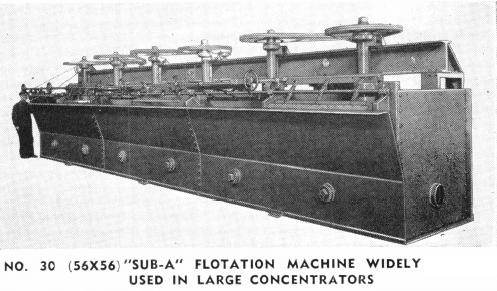
The field of industrial applications of flotation is just being scratched. Improved machines, reagents and technique are rapidly broadening the field for applications of flotation.
Order of Mineral Floatability
In the selective flotation process one sulphide mineral is floated in the presence of and without floating one or more other sulphide minerals. Although it is true that all sulphide minerals float, it is also true that the different sulphides have different floatabilities. Thus, under exactly the same conditions of machine, air bubbles, frothing oil, and time of floating, more of one pure sulphide mineral will be floated than of another. The order for the four common sulphides is copper, lead, zinc, and iron. Lead and copper are rather close together but zinc is decidedly lower in floatability and iron still lower. It may be correct to follow the rule whereby the easier a mineral particle oxidizes the harder it is to attach it to a bubble and float it. Usually the greater the luster which a particle has, the easier it will float. With these natural differences in floatability to work on and without the use of modifying or inhibiting agents, it is possible to selectively float lead or copper away from most of the zinc and iron by a deficiency of air, frothing oil, collectors, and time.
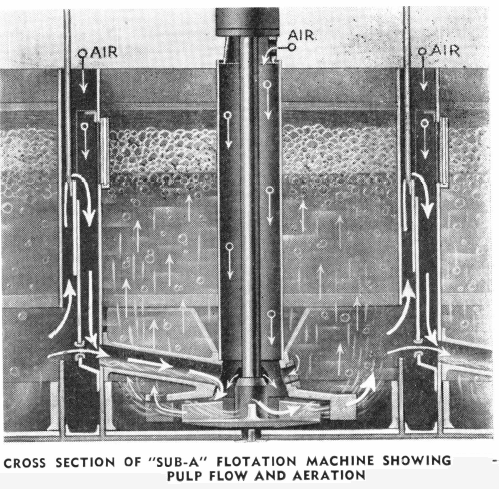
For successful selective flotation it was necessary to further modify the floatabilities of the sulphides. Also, it was desirable to increase the spread between the floatabilities of the sulphide to be floated, and those that were to be rejected or subsequently floated. This was accomplished by five methods, most of which are used almost universally today in the modern selective flotation mill:
- The use of collectors that are more selective in respect to the sulphide to be floated.
- The use of chemical modifying agents or inhibitors, such as lime or cyanide, which reduce the floatability of the iron or zinc sulphides without reducing that of the copper or lead.
- The use of conditioners which prepare the pulp for the flotation machine by giving the necessary time element for the chemicals and collectors to react with the mineral particles.
- The use of accurate reagent feeders which feed the exact amounts necessary to give the desired results.
- The use of the froth cleaning process in the flotation machine, which takes the rougher froth concentrate and retreats this concentrate to produce a cleaner concentrate that contains less of the sulphides not wanted and more of the sulphides wanted. The tailing from this cleaning step is returned usually to the rougher machine for retreatment. In some cases three or four cleaning operations are necessary to obtain the high grade shipping concentrate desired.
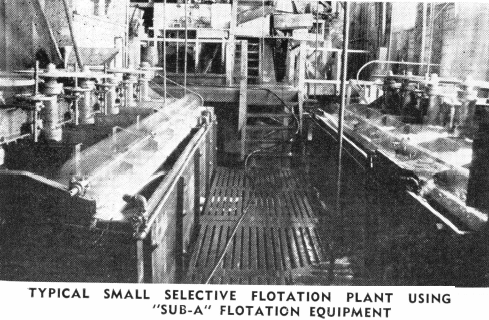
The “Sub-A” Flotation Machine has done much to further the successful use of selective flotation. The “Sub-A,” with its “cell to cell” gravity pulp flow and its method of cell design which permits the entrance of feed near the bottom of any cell, can be divided at the mill by a slight change in launders to any number of roughing, cleaning, and recleaning cells desired. This makes possible the highest grade concentrate and at the same time the retreatment of the middling products all in the same self-contained machine and without any additional pumps or elevators. This modern machine simplifies mill construction and operation and makes possible the best metallurgical results obtainable for selective flotation in the small mill as well as in the large one.

There is no branch of milling which has advanced as much as selective flotation during the last ten years, particularly as applied to non-metallic minerals. The recovery of as many as four or five minerals, in separate concentrates, is today quite common. Seldom are concentrates shipped which contain more than the one mineral. The mineral constituents are separated into individual products in many ingenious ways. The flotation machine has played an important part in this selective flotation practice. This is especially true of the cell to cell type of machine such as the “Sub-A,” where the number of cells to treat different minerals may be changed rapidly from time to time as contents and type of ore may vary. Modern and efficient reagent feeders—together with the vast variety of new and proven reagents—have also led to the spread of selective flotation into many new fields. In selective flotation it is better to use a large number of smaller cells in series than a few of the larger cells. The type of flotation machine is most important to make it possible to mechanically separate the desired froth from the remaining pulp. The design and location of the weir overflow separating the various cells is also very important.
In metallurgical plants more and more attention is being paid to the proper handling of middling products which are in many cases being subjected to separate grinding and subsequent treatment. The handling of these products has, at all times in the past, represented one of the most difficult problems in any mill. Study your middling circuit and you will answer most of your milling problems.
Leaching by Cyanidation
Although it was known in 1840 that cyanide solutions dissolved metallic gold, the process for ores was not developed until 1887 by the MacArthur Forrest Syndicate. Since that time it has been widely used for the recovery of gold and silver from certain types of ores. Recently its use in association with flotation and gravity concentration in many different combinations is replacing the straight cyanide process. This is especialy true in small plants where the initial cost is an important item in deciding on the mill flowsheet.
One of the most important of these modifications is the use of the Mineral Jig or the “Sub-A” Unit Flotation Cell placed in the ball mill-classifier circuit. Here these uits remove coarse free values, metallics, cyanicides, etc., and also coarse middling values for separate treatment. This modern step in the cyanide process results in saving in grinding costs and produces the same low tailing with much coarser grind.
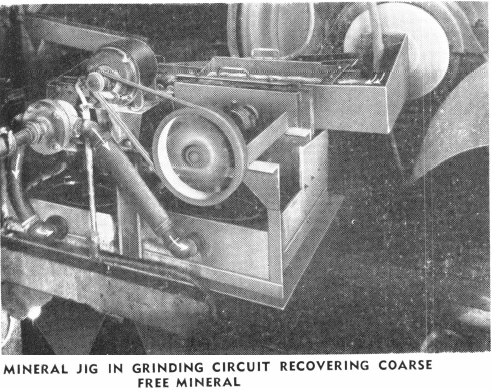
This method results in a saving in equipment and cost of subsequent agitation and thickening because the middling and coarse, free values that require a long treatment time have been removed previously from the circuit for separate treatment.
In the near future there will undoubtedly be increased activity in treatment of gold and silver ores. Amalgamation and cyanidation will play an important part in the recovery of these metals. There should be a great advance in the efficiency of methods for treating high grade gold and silver concentrates and also in recovering values from lower grade ores.
In past years it has been a common yard stick to assume that a cyanide plant must handle at least 100 tons per day to be profitable. Through the development of small continuous and batch cyanide plants, however, this view should be radically altered. These plants have a capacity of 10 to 25 tons per day (24 hours), and contain all the modern equipment required for amalgamation and cyanidation.
Improvements in standard grinding equipment, tanks, thickeners, pumps and filter design have made these modern plants practical. The marked advantage of being able to sample and mill ore from many parts of an extensive property are readily apparent.
Modern milling operations almost invariably consider exhaustive testwork on the ore to be an indispensable first step. Test equipment is not expensive, if it is desired to do the testwork on the scene of operation, or non-profit testing laboratories with experienced personnel, such as 911metallurgist.com may be called on to assist. In any event, complete testwork is a proved way to insure your investment.

Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
