Table of Contents
- Spectroscope to Identify RARE METALS
- Thallium by Spectroscopy
- Tungsten by Spectroscopy
- Rhodium by Spectroscopy
- Palladium by Spectroscopy
- Osmium by Spectroscopy
- Iridium by Spectroscopy
- Tellurium by Spectroscopy
- Selenium by Spectroscopy
- Molybdenum by Spectroscopy
- Beryllium or Glucinium Spectroscopy
- Zirconium Spectroscopy
- Thorium by Spectroscope
- Yttrium Spectroscopy
- Spectroscopy of Titanium
- Spectroscopy of Uranium
- Lithium by Spectroscope
- APPARATUS FOR Spectroscopic ASSAYING
- Spectroscopy Laboratory
FIG. 35 gives an idea of the spectroscope and of its different parts. P is a flint glass prism, having a refracting angle of 60° and resting on a brass plate fixed on a brass support, S. The brass plate carries the collimator tube C, in the end of which nearest to the prism is fixed a lens, the other end being closed by a plate in which there is a vertical slit, which can be widened or narrowed as required by means of a small screw
The tube E has also on the end nearest the prism a lens, and at the other end a reduced photographic millimetre scale which can be seen through the telescope T. At the end of E is placed an ordinary gas burner, a little distance from the photographic scale. Right opposite the slit in the collimator tube C is placed an ordinary bunsen flame, in which the substance to be tested is placed in the loop of a platinum wire. E is adjusted so that the image of the illuminated scale can be seen through the telescope T, and the divisions are focussed by means of a small screw on E. All extraneous rays of light are shut off by covering up the apparatus with a black cloth or experimenting in a dark room.

Fig. 36 gives the spectra of the alkalis and alkaline earths, and with care in adjusting the spectroscope a good many of these lines can be seen.
The chlorides of these metals are used for the analysis, but in the case of potassium the solid KNO3 is recommended to be used in the Pt. loop, as this brings out the violet line more distinctly.
The student should take all these salts in the order given, and in his note-book draw to scale each spectrum, only putting in the lines observed, then compare them with the chart.
Spectroscope to Identify RARE METALS
Thallium by Spectroscopy
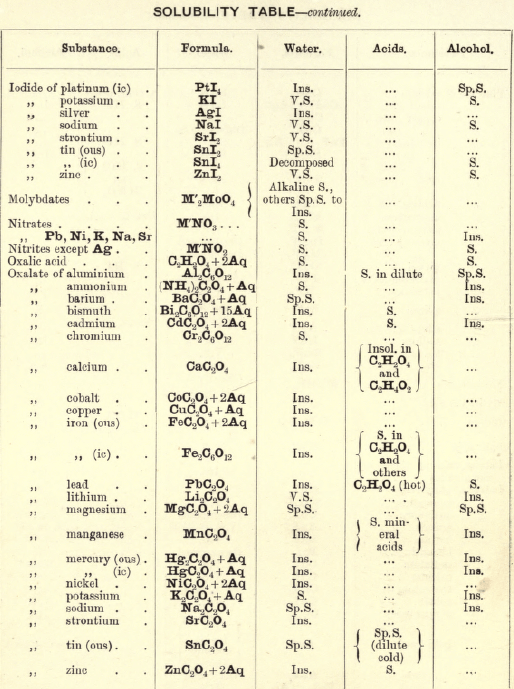
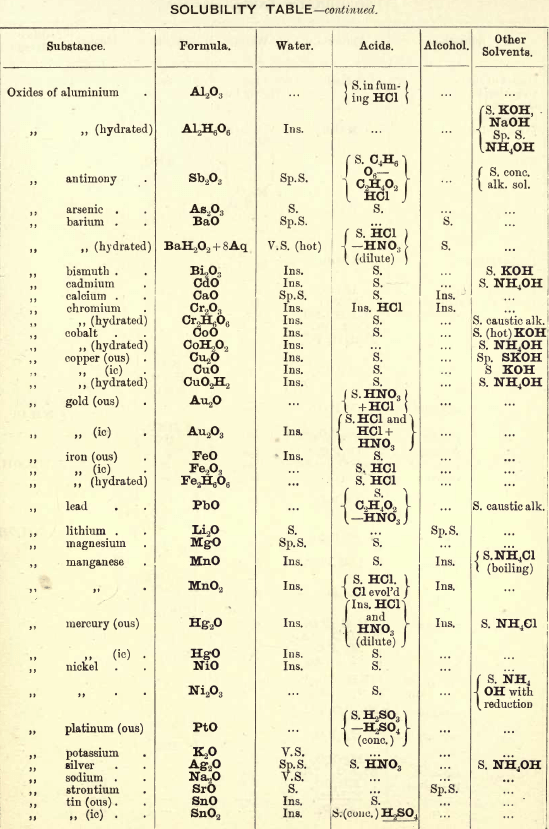
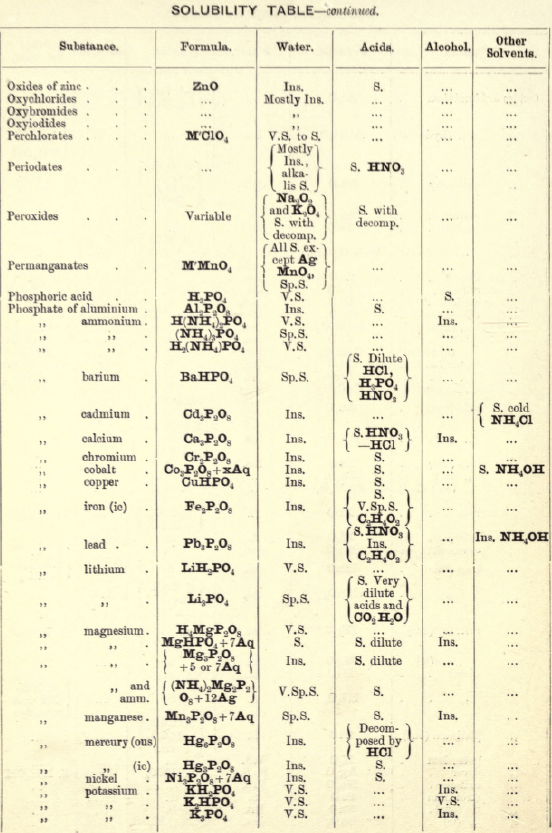
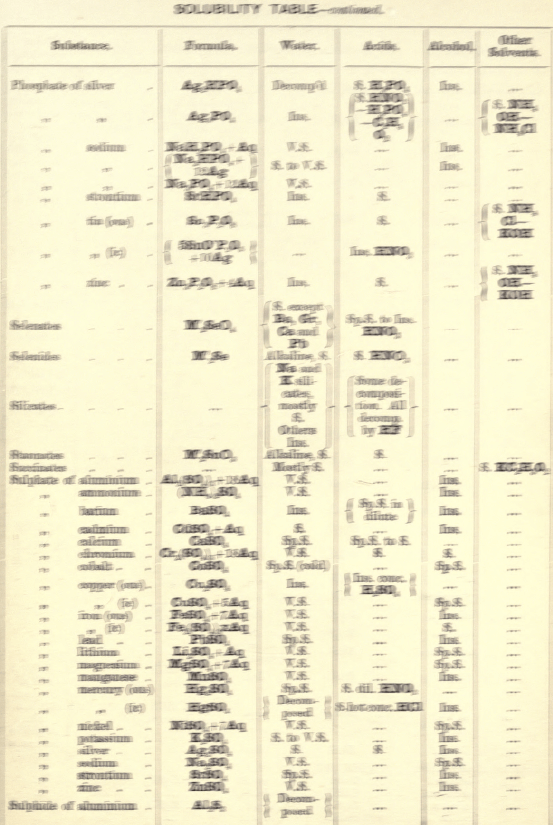
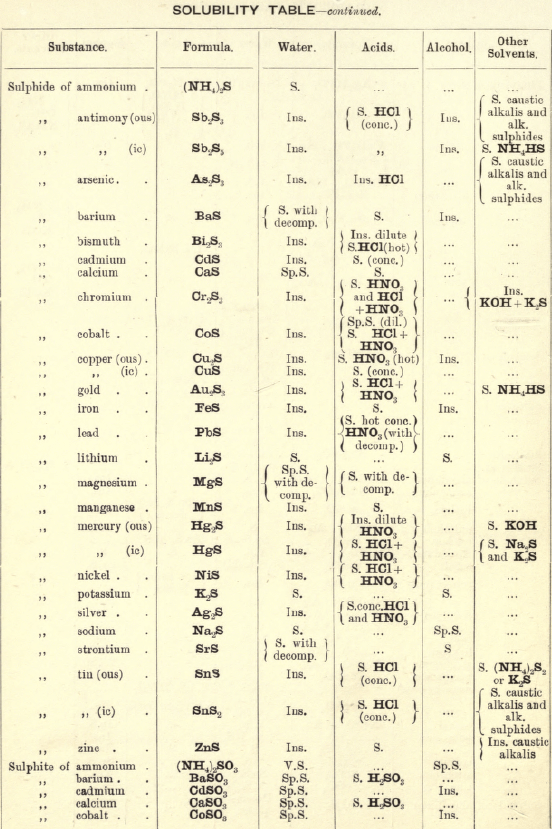
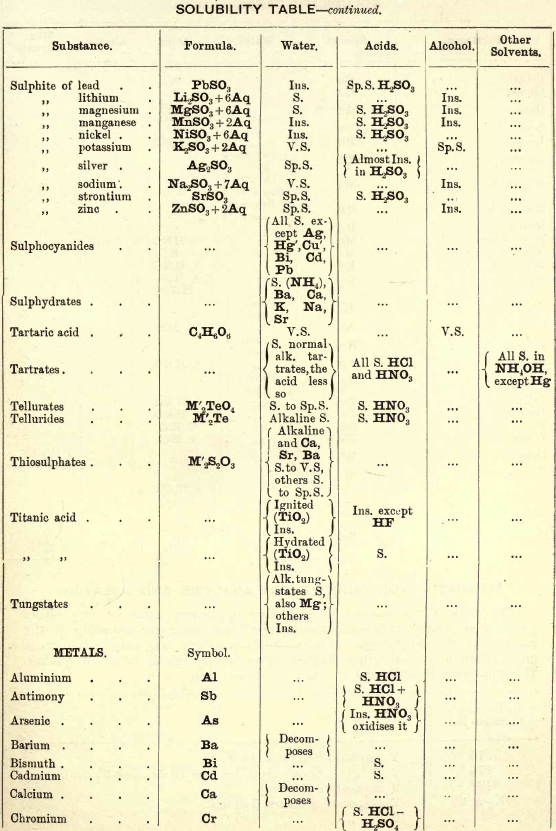
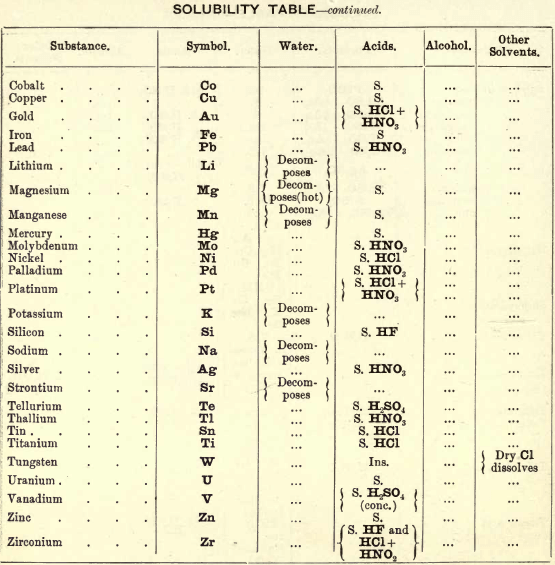
Soluble in HNO3. Tl2O is very soluble in H2O. HCl (conc.) precipitates TlCl (ous), white p’p’t, from aqueous solutions, slightly soluble in H2O, insoluble in NH4OH, but soluble in H2SO4.
H2S gives no p’p’t from mineral acid solutions, but from an acetic acid solution a brown p’p’t of Tl2S is thrown down. (NH4)2S also p’p’tes Tl2S.
PtCl4 p’p’tes from solutions of thallous salts, a yellow p’p’t of Tl2PtCl6.

Tungsten by Spectroscopy
Insoluble in HCl or H2SO4, slowly soluble in HNO3 or aqua regia. Mineral generally fused with Na2CO3 and NaNO3, when tungstates of the alkalis are formed which are soluble in H2O.
HCl in excess in an aqueous solution cold, gives a white p’p’t of H2WO4.H2O; hot, a yellow p’p’t of H2WO4.
H2S gives no p’p’t in acid solutions, but turns the solution a blue colour. A solution of a tungstate with Zn, Sn, or SnCl2, and an excess of HCl, gives a characteristic blue colour.
Gold and Platinum are insoluble in HCl, HNO3, or H2SO4, but soluble in aqua regia, yielding chlorides AuCl3 and PtCl4.
H2S gives black p’p’tes of AuS and PtS2 from chloride solutions in the cold, insoluble in HCl or HNO3, but soluble in aqua regia and (NH4)2S. (PtS2 is only partially dissolved in (NH4)2S in the presence of other sulphides.) In boiling solutions metallic Au is p’p’ted and not AuS. From the (NH4)2S solution, AuS and PtS2 are p’p’ted by the addition of HCl.
FeSO4 gives a brownish p’p’t of metallic Au with AuCl3 solution. If AuCl3 solution is weak, a bluish coloration only is seen. Pt is p’p’ted by FeSO4 only after boiling for some time.
SnCl2 gives a purple p’p’t (purple of cassius) in dilute solutions of AuCl3.
NH4Cl with AuCl3 and PtCl4 forms chloroaurates and chloroplatinates (NH4AuCl4 and (NH4)2PtCl6).
C2H2O4 p’p’tes Au but not Pt from chloride solutions.
To solution of AuCl3 + PtCl4 add C2H2O4

Rhodium by Spectroscopy
Alloyed with other metals Rh is soluble in aqua regia.
H2S.—From a hot solution of its salts, p’p’tes a sulphide insoluble in alkali sulphides.
Fused with KHSO4, and the fused mass treated with H2O, a pink solution is obtained. Fused with KOH and KNO3 gives RhO2, insoluble in acids and alkalis.
Melted with Zn and the alloy boiled with an acid, Rh is left as a black powder.
Palladium by Spectroscopy
Slowly soluble in HCl or H2SO4 ; dissolves in the cold in HNO3, forming Pd (NO3)2. Soluble in aqua, regia, forming PdCl4.
H2S p’p’tes it as PdS, insoluble in (NH4)2S. PdCl4 is very unstable, and is decomposed, even in solution, into PdCl2 and free Cl. KI p’p’tes black PdI2.
Pd salts are easily reduced to metal Pd either by heat or by the action of reducing agents.
Osmium by Spectroscopy
The metal in compact form is not attacked by acids. The p’p’ted metal is slowly dissolved by aqua regia or fuming HNO3. In alloys treated with aqua regia, and the residue heated in a porcelain tube through which air is passed, OsO4 (perosmic anhydride) is given off; a peculiar and irritating vapour, poisonous and injurious to the eyes. OsO4 is soluble in H2O, giving a neutral solution with powerful oxidising properties; bleaches indigo and liberates I from KI. Mixed with KCl and heated in Cl, the mass then treated with H2O and evaporated, red crystals of 2KCl,OsCl4 separate. H2S gives a brownish black p’p’t, OsS, in strongly acid solutions insoluble in (NH4)2S.
Iridium by Spectroscopy
Insoluble in acids and aqua regia. Freshly p’p’ted it is soluble in aqua regia. Mixed with NaCl and heated in a current of Cl. Na2IrCl6 is formed, soluble in boiling water. H2S reduces IrCl4 to Ir2Cl6 and p’p’tes Ir2S3 as a brown p’p’t, soluble in excess of (NH4)2S.
NH4Cl p’p’tes (NH4)2IrCl6 a dark red-brown p’p’t which upon ignition leaves metallic Ir.
Tellurium by Spectroscopy
Insoluble in HCl; soluble in HNO3 and aqua regia. H2SO4 (conc.) produces a purplish-red solution on cooling.
Fused with Na2CO3 on charcoal it forms Na2Te, which is decomposed with acids forming H2Te, a gas of very disagreeable odour.
H2S p’p’tes a brown sulphide soluble in (NH4)2S.
TeO2 dissolves in KOH and NaOH, forming tellurites.
Selenium by Spectroscopy
Soluble in HNO3 and aqua regia. H2SO4 (conc.) gives a green coloured solution on cooling, from which solution H2O p’p’tes it as a red powder. Fused with Na2CO3 on charcoal it forms Na2Se, which is decomposed with HCl and forms H2Se, a gas smelling of putrid horseradish.
H2S p’t’tes Se and S from selenious compounds, a lemon yellow p’p’t which becomes red on heating.
SeO2 is soluble in H2O.
Molybdenum by Spectroscopy
Insoluble in HCl and dilute H2SO4 converted into molybdic acid by HNO3.
MoO3 is soluble in strong NH4OH forming NH4HMoO4
HNO3 and HCl p’p’t H2MoO4 from concentrated molybdate solutions.
H2S from acid solutions, p’p’tes MoS3 a brownish-black p’p’t soluble in (NH4)2S.
(NH4)2MoO4 used for testing for H3PO4 (see General Table).
Beryllium or Glucinium Spectroscopy
Occurs generally as silicate with Al2O3 (beryl) or with Al2O3 (chrysoberyl). Mineral; fuse with four times its weight of (NaK)Co3, decompose fused mass with HCl and evaporate to dryness to separate SiO2. Take up with HCl, filter, and to the solution add excess of (NH4)2CO3, Al(OH)6 is p’p’ted. Be(OH)2 is soluble in the excess of (NH4)2CO3. Acidify solution with HCl and add NH4OH; Be(OH)2 is thrown down. BeO2 is soluble in acids.
Salts of Be have a sweetish taste.
Zirconium Spectroscopy
Occurs chiefly as silicate, ZrSiO4 (zircon). Mineral. Heat with KHF2 and boil with H2O. K2ZrF6 is dissolved; heat solution with H2SO4 to expel HF, and then p’p’te Zr with NH4OH as ZrO(OH)2.
ZrO(OH)2 is insoluble in excess of NaOH and KOH.
C2H2O4 p’p’tes Zr as oxalate, soluble in excess of (NH4)2C2O4.
Thorium by Spectroscope
Occurs chiefly as silicate (thorite). H2SO4 decomposes the mineral, and the sulphate of Th can he p’p’ted by boiling. C2H2O4 p’p’tes a white oxalate of Th, insoluble in dilute acids, soluble in NH4C2H3O2 + CH3COOH.
ThO2 can be obtained by igniting the oxalate.
Yttrium Spectroscopy
Occurs in rare minerals, such as Gadolinite, Orthite, etc.
Can be dissolved out by acids.
Yttrium salts are white.
NH4OH p’p’tes the hydrate insoluble in excess.
C2H2O4 p’p’tes the white oxalate of Y, insoluble in excess, partially dissolved by boiling with (NH4)2C2O4.
Spectroscopy of Titanium
Occurs generally as TiO2 in minerals, such as Ilmenite, Rutile, Anatase, etc. The mineral is fused with KHSO4 the fused mass is then treated with cold water and filtered. H2SO3 is added to the filtrate and it is boiled, when H2TiO3 separates out as a white p’p’t.
An acid solution of TiO2, when treated with Zn or Sn, gives a violet or light-blue colour to the solution.
Spectroscopy of Uranium
Occurs in nature in the minerals Pitchblende, Uranite, etc.
UO2 (ous), brown or black, dissolves in HNO3 and forms uranic nitrate (UO2(NO3)2).
UO3 (ic), brick red, dissolves in HCl.
Uranic salts (yellow colour) in H2SO4 solution, warmed with Zn, change colour of solution to a green colour; (NH4)2S from cold neutral solutions, in presence of NH4Cl, throws down a chocolate-brown p’p’t soluble in acids, including CH3COOH.
Lithium by Spectroscope
Occurs in mineral waters as Li2CO3, and in some minerals as silicate, such as Lepidolite, etc. Most of the lithium salts are soluble in water, the carbonate, hydroxide, and phosphate being less soluble than the others.
The chloride and nitrate are soluble in a mixture of alcohol and ether, which distinguish it from Na and K.
Na2HPO4 p’p’tes Li3PO4 as a white p’p’t on boiling. P’p’tion more complete in the presence of NaOH.
Li salts impart a deep carmine colour to the flame.
Spectrum— one red and one orange line.
https://www.youtube.com/watch?v=2tZ6plRHLMg
MINERALS AND ALLOYS FOR QUALITATIVE ANALYSIS
Minerals.—Calcite, dolomite, magnesite, siderite, apatite, fluorite, barytes, celestine, felspar, galena, blende, iron and copper pyrites, stibnite, smaltine, fahl ores, bismuthinite, calaverite, chromite, wolfram, topaz, beryl, tourmaline.
Alloys.—Brass, Muntz metal, bell metal, Britannia metal, soft solder, phosphor bronze, rose metal, aluminium bronze, German silver, steel.
At least six of the above minerals and four of the alloys are recommended to be analysed by each student, and should be selected by the demonstrator.
Before proceeding to the analysis of minerals and alloys the student should be thoroughly conversant with all the qualitative tables and should be able to apply them without any difficulty. Too much importance cannot be placed on the preliminary tests, as most of the bases should be detected there first and afterwards confirmed in the wet way.
A careful qualitative analysis should always be made of a substance before the quantitative is begun, as the methods to be adopted in the latter greatly depend upon what is present.
APPARATUS FOR Spectroscopic ASSAYING
The student will require the following apparatus, which he may either purchase or obtain on deposit, making good any breakages according to the practice of the laboratory. Gallenkamp’s numbers are again given as a guide, though several other English firms supply goods of equal quality This apparatus is additional to what he already has.

Spectroscopy Laboratory
Balance.
Water oven, air oven.
Hydrometers.
Diamond mortar, sieves, agate and steel mortars.
Hot plates, combustion furnace and fittings.
All the more complicated glassware and other apparatus.
Furnaces, grinding appliances, furnace tools, gold rolls, etc., required in assaying.
Any special apparatus required in technical analysis.
The student may procure or obtain on deposit:
1 box gram weights.
1 box assay weights.
Platinum crucible (30 c.c.).
Also it will be more convenient if arrangements be made to obtain all crucibles, scorifiers, and other assaying stores from the laboratory stores as required, as such materials take up too much of his locker space. Such stores to be given out once a week.
The above lists include most of the ordinary requirements. Any additional apparatus may be procured during the course.
