Table of Contents
Bacterial attack of sulfide minerals has been going on for centuries but only recently was it discovered that the acid and iron often found in coal mine drainage is produced by bacterial action on the iron sulfides in the coals. Not until 1950 was it proven that bacteria were playing a role in the dump leaching of sulfide ores.
Research to Date
Thiobacillus ferrooxidans derives its energy from the oxidation of ferrous iron and reduced sulfur compounds. Contrary to the chemical reactions involved in oxide leaching, the reactions occurring during bacteriological oxidation are rather complex and environmental conditions greatly affect the activity of the bacterium.
(a) Chemically, by ferric iron with the bacteria reoxidizing the ferrous iron to the ferric form.
(b) Direct microbiological oxidation of the sulfide.
Indirect
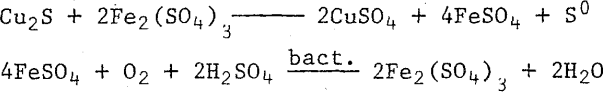
Direct
![]()
Conclusive proof for direct microbiological attack on the sulfide portion of the minerals was obtained in a study conducted in our laboratory using enzyme inhibitors. When the enzymes controlling iron oxidation and sulfide oxidation were selectively inhibited and the rate of leaching measured, it became evident that Thiobacillus ferrooxidans could break down a sulfide mineral when it was unable to oxidize ferrous iron.
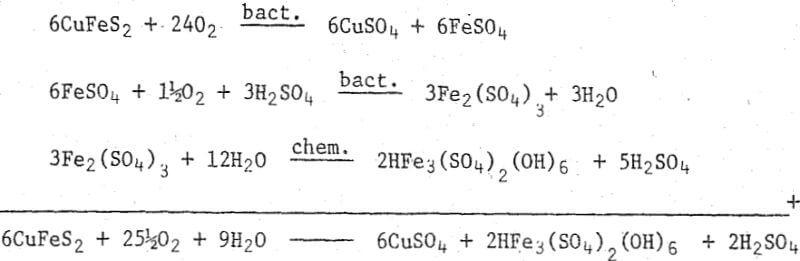
This reaction is acid-producing and water-consuming, due to the precipitation of ferric iron as basic ferric sulfate, a jarosite type of mineral. In our experience, the oxidation of the iron and its subsequent precipitation is so rapid that we believe it occurs simultaneously with the sulfide oxidation.
Bacterial Nutrients
The leaching bacterium is a living organism and certain nutrients are required for its metabolic systems. Most importantly, nitrogen is required for the production of body protein, but unfortunately the bacteria are unable to utilize nitrogen from the air or nitrate nitrogen. The usual source of supply is ammonium ion. Other nutrients required are phosphate, manganese, potassium, calcium and magnesium. Carbon dioxide is the sole source of carbon for the bacterium, which is unable to utilize organic carbon.
The significance of nutrition to these bacteria is made evident when it is realized that the microbiological release of metal increases with increasing cell division which, in turn, is associated with bacterial growth. When growth ceases, metal release is practically zero.
Examination of leach liquors obtained from many different leach dumps has always indicated the presence of some phosphate and maximum extractions were obtained, although at slow rates. Considering the slow rate of metal release occurring in a leach dump, we do not currently consider the addition of phosphate necessary unless chemical analysis shows it to be completely absent.
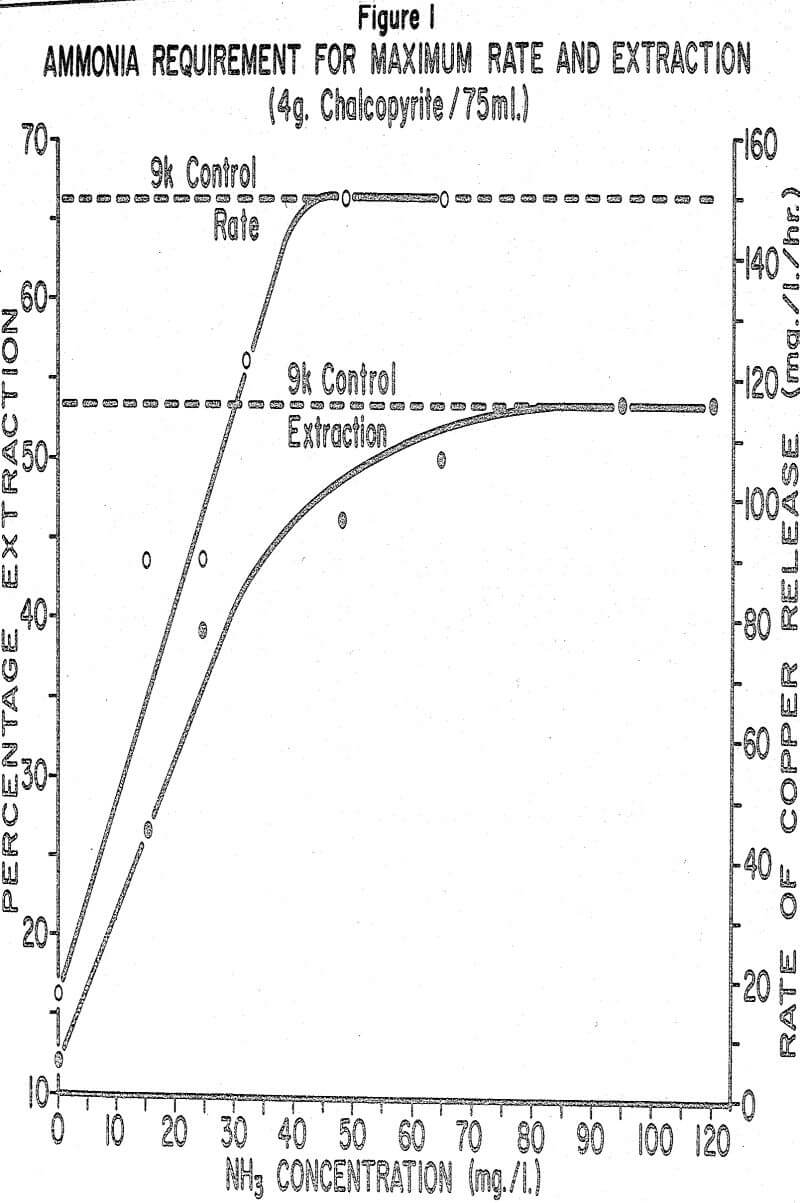
If we change our thinking now to the operation of a large dump where millions of tons of low-grade mineralization are subjected to bio-logical leaching, it can be understood that even at very low levels of activity large amounts of copper can be extracted from the dump. However, it is quite probable that more copper could be extracted more rapidly if more ammonia was made available to the bacteria.
Based on the results of our research work we strongly recommend that all the liquors utilized in dump, heap or in-place leaching be analyzed for ammonia content and, wherever necessary, ammonium be added to the leach liquor.
Current Leaching Results
The application of our leaching knowledge resulted in considerable improvement in chalcopyrite leaching rates. From an initial rate pf below 25 mg/l/hr in 1962, we had increased the copper release rate to 250 mg/l/hr by 1966 and presently we have had rates as high as 700 mg/l/hr. Considerably higher rates, ranging from 700 to 1300 mg/l/hr, have been obtained while leaching zinc sulfide.
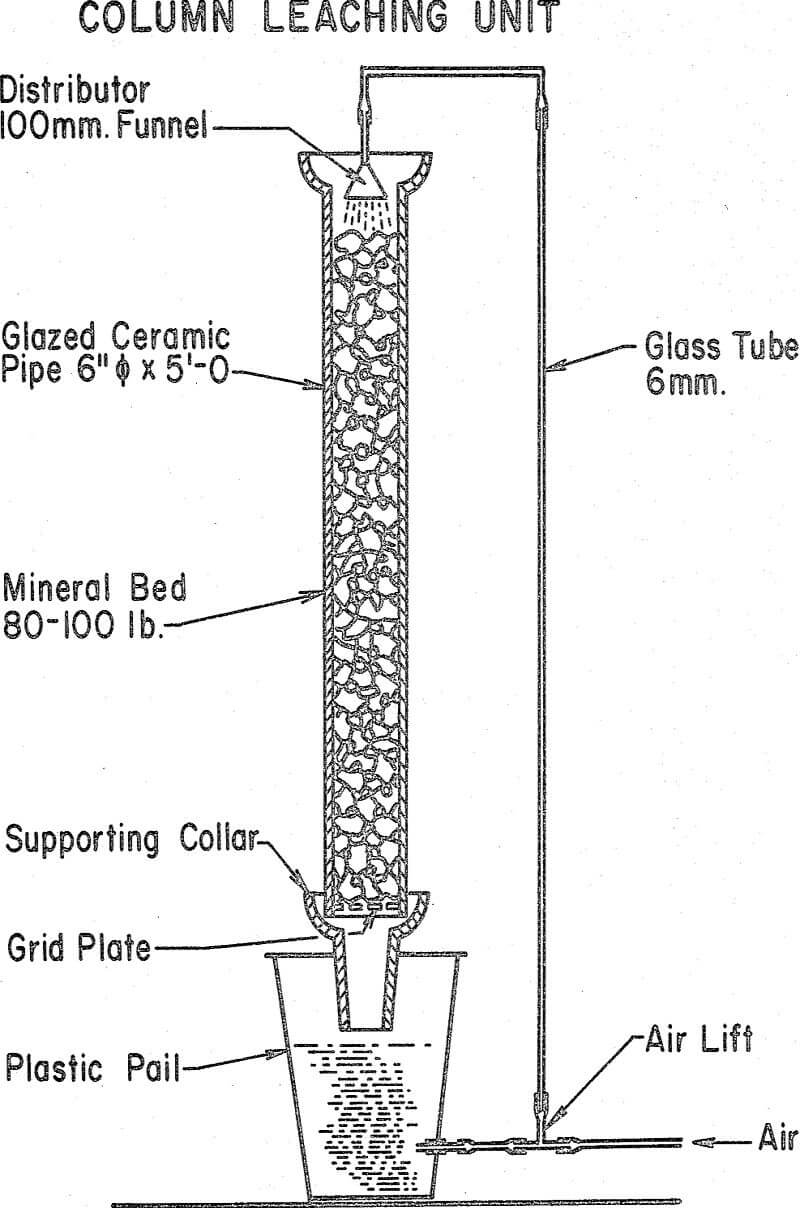
Since the leaching rate is the function of the availability of the sulfide mineral, it stands to reason that rates obtained during column studies will be slower than when leaching finely-ground concentrate. The concentrate leach curve shows the typical lag phase during which the bacteria adapt to the environment. After a 24-hr lag, cell division starts and, since cell division is proportional to sulfide oxidation, the copper released becomes noticeable. The curve shows that the growth is proceeding on an exponential rate until, after 96 hours, the release of copper becomes linear, probably because the rate of oxygen transfer from the atmosphere cannot meet the bacterial demand.
We realize of course that a dump cannot be operated under the same ideal conditions as used in our column studies but there can be no question that substantial improvements are possible when the bacterial environment is changed towards the optimum pH, ammonia level and oxygen supply.