Table of Contents
- Reagent Preparation
- Preparation
- Standardization
- Calculation
- Preparation
- Standardization
- Sodium Diphenylamine Sulfonate Indicator
- Vanadium-Acid Mixture for Ferrous Iron
- Silver Nitrate Solution
- Stannous Chloride Reducing Solution
- Saturated Mercuric Chloride Solution
- Manganese Oxidant Solution
- Saturated Manganese Solution
- Zinc Oxide Slurry
- Sample Preparation
This report describes the elemental characterization of chromite and related materials at the Bureau of Mines, Albany Research Center. Analytical methods for determining the major constituents, representing extensive experience, refinement, and development, are described and fully annotated. This presentation should allow other laboratories to use these methods and obtain comparable results with a minimum of time needed for familiarization with individual methods.
A goal of the Bureau of Mines is the maintenance of an adequate supply of critical minerals and metals to meet national economic and strategic needs. As part of this effort, the Bureau’s Albany Research Center has conducted research related to chromium ores and production. In providing analytical support, the Center has analyzed many samples of chromium materials and has acquired considerable experience in the requisite techniques.
Chromium is on the critical materials list because it is widely used in strengthening and enhancing the corrosion resistance of ferrous alloys and there is a lack of adequate domestic resources. In general, the United States has relied on imports to supply 91 pct of its chromium needs, depending on secondary recovery to supply the remaining 9 pct.
Research projects at the Albany Research Center have ranged from the location and characterization of domestic sources of chromite ore, through smelting ferrochrome ingot operations, to the recovery of chromium and chromium-bearing minerals from various industrial wastes. Reports published include Bureau of Mines Information Circular 8916, entitled “Podiform Chromite Occurrences in the Caribou Mountain and Lower Kanuti River Areas, Central Alaska. Part II: Beneficiation” and Bureau of Mines Report of Investigations 8676, entitled “Chromium Recovery From Nickel-Cobalt Laterite and Laterite Leach Residues”.
In support of these and other research projects, this Center has analyzed a large number of samples of chromite ore, ferrochrome, ferrochrome slag, and solids and solutions from chromium recovery investigations. The large number of samples processed has promoted refinement of the analytical methods used, so that reliability of the results is not sacrificed in order to return the data in a timely manner. The results of this refinement are presented in this publication.
The purpose of this report is to present the techniques used at the Albany Research Center for the analysis of chromite minerals and ferrochrome-related samples. The methods given in this report are based on standard techniques. The presentation is concise and offers supplementary information to aid the understanding of less experienced analysts and technicians. Toward this goal, the format for the presentation of each method includes:
- A short introductory note on each method.
- Lists of equipment and materials.
- A step-by-step listing of the procedure.
- Procedure notes that provide information pertaining to the reasons for a step in the method or supplementary instructions referring to indications of success, malfunction, or optional actions that may be taken.
The preparation of reagent solutions is presented in appendix A. Appendixes B and C present some notes on the use of zirconium crucibles and on sample preparation.
This format should allow the experienced analyst to take this report into the laboratory and produce results that are comparable and compatible with results obtained at this Center, with a minimum of delay for familiarization with the techniques, and this format should accelerate the understanding of the less experienced analyst.
- Chromium in Mineral Chromite & Ferrochrome Slags
- Acid-Soluble or “Metallic” Chromium in Ferrochrome Slags
- Total Iron in Mineral Chromite and Ferrochrome Slags
- Ferrous Iron in Mineral Chromite and Ferrochrome Slags
- Metallic Iron in Ferrochrome Slags
- Aluminium and Magnesium in Mineral Chromite and Ferrochrome Slags
- Calcium in Mineral Chromite and Ferrochrome Slags
- Gravimetric Silica in Mineral Chromite and Ferrochrome Slags
- Manganese in Mineral Chromite, Ferrochrome Slags, and Ferrochrome
- Acid Digestion of Ferrochrome Metal
Analysts assigned to make a new determination on a sample often must deduce the significance of steps in the usually sketchy procedure descriptions of in-house methods and methods in journal or reference publications. The familiarization process results in a delay in mastering the method. This report seeks to shorten that delay, allowing analysts newly assigned to the covered determinations a quicker understanding of the methods.
The information presented here was collected empirically by the analysis of thousands of samples over several years and the observation of the effects of small variations in technique.
The reliance on zirconium crucibles as alkaline fusion vessels may have been noted. Their reliability in terms of durability and lowered introduction of interfering species in comparison to iron and nickel crucibles has been proven to the satisfaction of this Center. They should not, however, be used in fusions in a muffle furnace because of significant oxygen attack.
Any request for assistance concerning the subject of this report should be addressed to the authors, Bureau of Mines, Albany Research Center, Albany, OR.
Reagent Preparation
0.1N Fe+2 Solution
Preparation
Dissolve 39 g of ferrous ammonium sulfate hexahydrate in distilled water with 10 mL of concentrated sulfuric acid. Dilute to 1 L with distilled water.
Standardization
Weigh accurately 130 to 140 mg of primary standard- grade potassium dichromate into each of three 400-mL beakers. Add about 150 ml- of distilled water and about 5 mL of concentrated sulfuric acid, and stir until completely dissolved. Titrate with the new ferrous solution to the disappearance of the diphenylamine sulfonate indicator purple.
Calculation
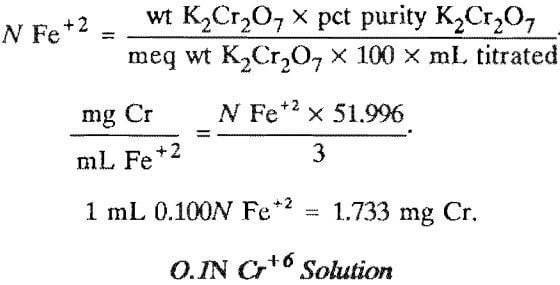
Preparation
Dissolve 4.9 g of potassium dichromate in distilled water and dilute to 1 L.
Standardization
Weigh accurately 0.17 to 0.18 g of electrolytic or NBS standard-grade iron metal into each of three 250-mL beakers. Add 20 mL of distilled water and 20 mL of concentrated hydrochloric acid, cover, and heat gently until the iron is completely dissolved. When dissolved, remove from the heat. Remove the cover, and rinse any condensate into the beaker. Rinse the beaker sides, and quantitatively transfer the solution to a 500-mL Erlenmeyer flask. Make the volume, up to 125 to 150 mL with distilled water. Bring to a boil and, dropwise, add 10-pct stannous chloride solution, with mixing, until the solution is colorless. Add 3 to 5 drops excess and just bring to a boil again. Cool to room temperature or below, and add 10 mL of a saturated solution of mercuric chloride: mix, and add 10 mL of concentrated phosphoric acid. Titrate with the prepared Cr*6 solution using sodium diphenylamine sulfonate indicator to a purple endpoint.
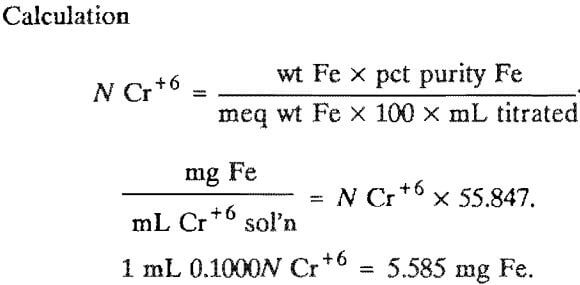
Sodium Diphenylamine Sulfonate Indicator
Dissolve 1.35 g of reagent-grade sodium diphenylamine sulfonate in 500 mL of distilled water.
Vanadium-Acid Mixture for Ferrous Iron
Add 3.0 g of vanadium pentoxide (V2O5) to a mixture of 300 mL of concentrated phosphoric acid and 600 mL of concentrated sulfuric acid. Heat and stir until all of the vanadium pentoxide has dissolved. Add, dropwise, a saturated solution of potassium permanganate until the acid mixture gives a faint pink when a few drops are diluted with water. Heat to fumes of sulfuric acid, and continue to fume for several minutes to destroy the excess permanganate. Cool thoroughly and transfer to a stock bottle. If filtration is necessary, use a pad of glass wool in a large funnel.
Silver Nitrate Solution
Dissolve 25 g of reagent-grade silver nitrate in distilled water and 10 mL of concentrated nitric acid. Dilute to 1 L and store away from light.
Stannous Chloride Reducing Solution
Dissolve 20 g of stannous chloride in 200 mL of 20-pct (1:4) hydrochloric acid. Make this solution fresh weekly or store it in a bottle with several high-purity tin shot.
Saturated Mercuric Chloride Solution
Heat distilled water in a large beaker to almost boiling. Add reagent-grade mercuric chloride to the water by the spoon or spatula with vigorous stirring until no more will dissolve. Transfer to a stock bottle.
Manganese Oxidant Solution
Dissolve 7.5 g of reagent-grade potassium periodate in 100 mL of hot 1:1 nitric acid. When dissolved, cool and add 400 mL of concentrated phosphoric acid, dilute to 1 L, and transfer to a stock bottle.
Saturated Manganese Solution
Nearly fill a convenient size stock bottle with warm distilled water. Add manganese sulfate or potassium permanganate to the bottle, and shake or stir until dissolved. Repeat until no more will dissolve, and add a small excess. Transfer a portion of the supernatant liquid to a dropping bottle for use. Replenish the water or the manganese compound as necessary.
Zinc Oxide Slurry
Half fill a 250-mL beaker with zinc oxide powder. Add distilled water with stirring until the slurry is smooth and is the consistency of heavy cream or heavy latex paint. Stir the slurry occasionally during use.
Sample Preparation
The grind size or mesh size of the sample is an almost crucial consideration in the analysis of mineral chromite, ferrochrome slags, and ferrochrome metal. In general, the finer the sample particle size, the easier it will be to dissolve. If all samples could be ground to pass a 200-mesh screen, nearly all of the dissolution problems would be solved. In practice, a sample ground to pass a 100-mesh screen will present little in the way of problems, but for mineral and slag samples in particular, larger sample particles should be avoided. Larger sample particles of metal, instead of being harder to fuse, are actually easier to fuse but have a tendency to produce hot spots in the crucible and so contribute to burnthrough. Larger metal particles, of course, are slower to dissolve in acid than smaller particles. As a general rule, any sample ground to pass a 100-mesh screen should be acceptable, and any sample that will not pass a 60-mesh screen will present problems with sample decomposition.
