Table of Contents
This term is usually defined as “the alkaline hydrates and half the monocarbonates” whose action is to protect the cyanide from decomposition, by acids developed in the ore and by atmospheric carbon dioxide.
Either oxalic acid or a mineral acid may be used as a standard solution. Objections have been raised to the use of oxalic on the ground that it is unstable in weak solutions. This is no doubt true where standard solutions are to be kept in stock for an indefinite time, and where extreme accuracy is required, but it has been proved by the writer that decinormal oxalic acid may be kept in a bottle ¾ full of air in a tropical climate for at least 4 weeks without showing any appreciable deterioration when checked against carefully standardized decinormal alkali. The advantage of oxalic is that if a good brand be used and the bottle of crystals be kept well stoppered, it may be weighed up, dissolved, and used without standardization. Of course, theoretical accuracy is not attained without standardization on account of uncertain hydration of the crystals, but with the precautions mentioned such a solution is perfectly adequate for all purposes of alkali control in plant solutions.
The decinormal solution is made by dissolving 6.3 grams of the oxalic acid crystals in distilled water and making up to a litre. Decinormal solution of nitric or some other mineral acid may be used if preferred, but it is necessary that this should be standardized against a standard alkali. For this purpose Sutton recommends sodium carbonate as being the most useful. To make the decinormal solution take 10 or 12 grams of pure sodium carbonate, ignite gently, and cool under the desiccator. Then quickly weigh up 5.3 grams of this and dissolve in hot distilled water. When cool put into a litre flask and fill up to the mark with distilled water.
A decinormal nitric acid solution contains 6.3 grams of HNO3 per litre. If the acid is about 1.4 sp. gr. put 6.5 cc of colorless acid into a litre flask and fill up to the mark with distilled water. A given volume of the decinormal soda solution is taken (say 50 cc), and a few drops of methyl orange (not phenolphthalein) indicator added. The nitric acid solution is then run in from a burette until the color of the indicator shows that the solution is neutral. If the nitric acid solution is decinormal it should take exactly 50 cc of it to neutralize the soda. If it needs more or less than 50 cc it is incorrect, and the strength may be rectified by adding water or acid, as the case may be, after calculating the amount necessary. It should then be verified by another titration.
As an indicator for determination of protective alkali phenolphthalein is generally used, as it gives a value for monocarbonates corresponding with the definition of protective alkali already mentioned. Moreover, methyl orange cannot be used as indicator with oxalic acid. Dissolve about 0.5 gram of phenolphthalein in a little alcohol and add water up to 100 cc. Should a milkiness form on adding water, more alcohol is needed.
Two methods are in general use for determining protective alkali, (1) Clennell’s, and (2) Green’s.
Clennell’s Method for Protective Alkali
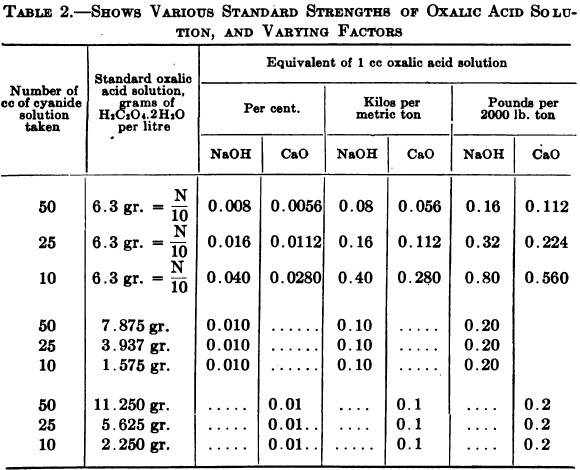
This is carried out on the same portion of solution that was used for the free cyanide test, method No. 2. After the necessary amount of silver nitrate has been run in to determine free cyanide a few drops of the phenolphthalein indicator are added, giving a rose pink color to the solution if free alkali is present; it is then titrated with the decinormal acid until the pink color is dispelled. Taking 25 cc of original cyanide solution, the number of cc of N/10 acid used X 0.016 = % free alkali in terms of NaOH, or X 0.0112 = % in terms of CaO. If 10 cc of original cyanide solution was taken, multiply the number of cc of N/10 acid used by 0.04 to get percentage of alkalinity in terms of NaOH or by 0.028 to get percentage of alkalinity in terms of CaO.
H2C2O4.2H2O + 2NaOH = Na2C2O4 + 4H2O
Atomic weights: 126 oxalic = 80 sodium hydroxide.
Decinormal oxalic acid = 6.3 grams per litre, equivalent to 4.0 grams of NaOH. 1 cc N/10 acid = 0.0063 gm. = 0.004 gm. NaOH.
If 25 cc of mill solution be taken for the test then 1 cc N/10 acid = 0.004 gm. NaOH = 0.016%, therefore No. of cc N/10 acid X 0.016 = % NaOH.
Special standard acid is frequently used in preference to N/10.
- To Read in Terms of NaOH —If 3.937 grams of oxalic acid crystals be dissolved and made up to 1 litre with water, then, taking 25 cc of original cyanide solution the number of cc of standard acid used X 0.01 = % free alkali in terms of NaOH, or X 0.1 its equivalent in kilos of NaOH per metric ton or X 0.2 = its equivalent in lb. of NaOH per ton of 2000 lb.
If 10 cc of original cyanide solution be taken, the special solution is made by dissolving 1.575 gm. of oxalic acid in water and making up to a litre In this case 1 cc of acid = 0.01% NaOH.
- To Read in Terms of CaO.—If 5.625 grams of oxalic acid per litre be used, then the number of cc X 0.01 = % of free alkali in terms of CaO, or X 0.1 = its equivalent in kilos per metric ton or X 0.2 = its equivalent in lb. of CaO per ton of 2000 lb.
If 10 cc of original cyanide solution be taken the special solution is made by dissolving 2.25 gm. oxalic acid per litre, 1 cc of which = 0.01% CaO.
This method is perfectly satisfactory in solutions containing zinc, provided that the preliminary titration for free cyanide given under method No. 2 has been carefully performed and addition of silver has been stopped before more than the merest trace of zinc has been precipitated. Green has shown that as soon as sufficient silver has been added to throw down a precipitate of zinc there is a formation of acid zinc nitrate which destroys the reliability of the test for alkali.
Green’s Method for Protective Alkali
In order to avoid the risk just mentioned Green devised the following method. First determine total cyanide, as described, recording the number of cc of silver nitrate used. Take a new 25 cc of the solution to be tested and add an excess of potassium ferrocyanide (usually 5 to 10 drops of 10% solution will suffice), then run in from the burette the number of cc of silver nitrate recorded in the preliminary test, and a few drops over. Then add phenolphthalein indicator, and titrate with standard or decinormal acid. Calculate protective alkali as in Clennell’s method.
How to Measure the Available Alkalinity of Lime
The sample as delivered for analysis is contained in an air-tight vessel, having been passed through a 30-mesh sieve. It is crushed with a Wedgewood mortar and pestle, and the whole passed through a 60-mesh sieve, this operation being performed as quickly as possible. It is then placed in a clean, dry, wide-mouthed bottle fitted with a tight-fitting dry glass-stopper so as to prevent any access of air. Two grams of the sample is carefully weighed out and agitated with 1 litre of a 2 per cent, cane-sugar solution (or 1 gm. with ½ litre of a 2 per cent, sugar solution). If a shaking machine be available, 2 hours continuous agitation should be given, if not, 6 hours intermittent agitation, every care being taken to prevent coagulation of the lime, in order that the lime and solution may be brought into the most intimate contact during this period. When the agitation is finished the solution is filtered as quickly as possible, and aliquot portions titrated with N/10 or N/5 acid, using rosolic acid as indicator, avoiding delay so as to obviate undue exposure to the atmosphere. The distilled water used in the above determination must be made neutral to rosolic acid to counteract the presence of dissolved CO2.
