The present work was undertaken to develop a method to cathodically protect a ball mill to reduce materials loss from mill liners. The essential science of “Cathodic Protection” is to apply a sufficiently negative potential to a metal in a corrosive environment such that the anodic corrosion rate is reduced to a negligibly small or acceptable value. The metal is maintained in the active corrosion regime (as opposed to passive regime) but the overpotential for anodic dissolution is reduced by galvanic protection to the point that little corrosion occurs.
Until now studies of mechanisms of grinding media and metal loss in ball mills have been simulated using a rotating cylinder/anvil apparatus or a rotating ball/pin apparatus. The present work is conducted on a bench scale ball mill under operating conditions. Weight loss measurements were conducted on coupons inserted flush with the interior surface of the mill. Cathodic protection has been applied to both the coupons and to mill shell. Synergistic effects and pH effects on materials loss were also studied.
Experimental Details
Figure 1 shows a schematic diagram of a cross sectional view of the ball mill used for this study. It was constructed using a 25 cm diameter mild steel pipe with a wall thickness of 1 cm and a length of 35 cm. Eight lifter bars of 1 cm radius were fixed at regular intervals for efficient grinding. The mill rests on two rollers that are driven by a ¾ h.p, three phase motor.
Four mild steel coupons were inserted and set flush with the interior surface of the mill. These coupons were of 2.5 cm in diameter and 1 cm thick. They were electrically isolated from the mill with rubber grommets. The potential of each coupon could be controlled through carbon brushes riding on copper rings placed concentric to the mill and electrically connected to the coupons. (Eventually carbon brushes were replaced by copper rings due to the high resistance and noise associated with carbon brushes). Total liner wear was estimated based on the weight loss of the coupons after grinding. Two separate copper rings and brush assemblies were also attached to the mill shell to control the potential of the entire mill.
The mill was housed in an airtight plexi-glass chamber in order to inert the system. The slurry flowed out of the mill outlet into a sump, from which it was pumped peristaltically at a rate of 0.5 l/min into the anodic sump placed close to the mill inlet. Thus the slurry was recirculated during the entire test period (75 minutes).The mill was run at a speed of 60 rpm, which is 67% of the critical speed.
A Titanium-Indium Oxide dimensionally stable anode (DSA), supplied by Electrotech corporation was used for cathodic protection. The anode was placed in an anode sump and isolated from the slurry with a high density polyethylene membrane (HDPE) to prevent particulates from reaching the anode. The slurry provided a continuous ionic conducting path between the anodic sump and the interior of the mill. Any gaseous oxygen produced at the anode was released through a vent in the anode compartment.
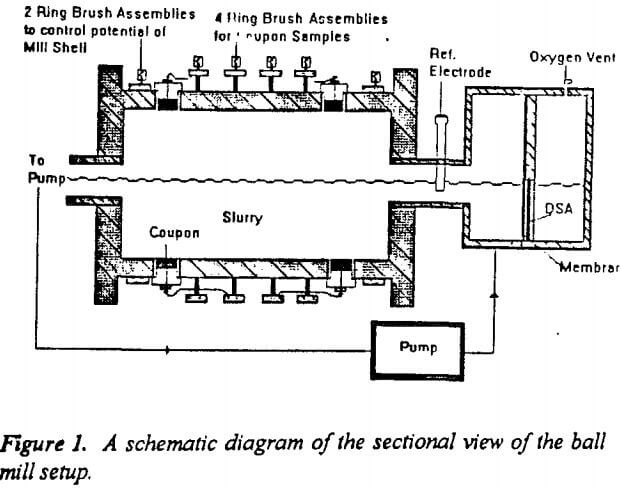
The potential applied to the coupons was controlled using an EG&G PARC Model 273 potentiostat. A low voltage, high current power supply (10V, 50 A) with adjustable current and voltage outputs was used to cathodically protect the entire mill shell. An industrial SCE (standard calomel electrode) was used as the reference electrode. The SCE was placed in the pipe between the anodic sump and the mill.
All grinding tests were carried out using quartz as an abrasive non-conducting mineral and pyrite as a conducting sulfide mineral. The quartz and pyrite used for this study were obtained from Bull quart vein, Floyd county, VA, and Morococha, Peru, respectively. A major part of this study has been conducted using ceramic balls as grinding media. Emphasis of the work has been on liner wear and liner-mineral interaction. The influence of different types of gaseous environment namely nitrogen, and oxygen on liner wear was established. In addition rest potentials and galvanic potentials for mild steel-pyrite electrodes were measured.
Effect of Cathodic Protection on Ball Mill Shell
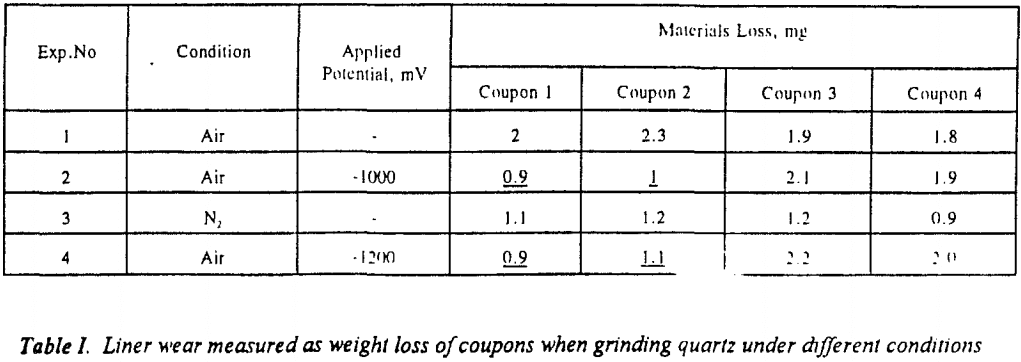
In the above studies cathodic protection was applied to test the coupons only. Attempts were made to protect the entire mill shell cathodically. A low voltage, high current (10 V, 50 A) power supply was used for this purpose. A potential of 3 V was applied between the anode and the mill. Two of the coupons were protected by electrical connection to the mill shell; the other two were left floating. Ceramic balls were used as the grinding media. Table VI shows weight loss measurements obtained while protecting the entire mill. Tests 2 and 4 indicate cathodic protection to be very effective in reducing materials loss by 40 to 50 % when grinding quartz and pyrite. This result suggests that cathodic protection can be used directly in an industrial ball mill to reduce liner wear appreciably.
Cathodic protection was used effectively to reduce materials loss in tumbling mills by 30 – 50 %. Although pyrite is a less abrasive mineral than quartz, it, caused more materials loss than quartz, indicating the importance of corrosion due to galvanic coupling in tumbling mills. The corrosive wear was found to increase with decreasing solution pH, possibly due to contributions from pitting. When grinding pyrite materials loss due to synergistic effect between abrasion and corrosion is higher when grinding pyrite than quartz. Surface analysis of the coupons indicated that the amount of iron oxide formed on the surface decrease with cathodic protection.

