Table of Contents
The correlation of the high temperature chemical properties of slag-metal systems with some easily measured property of either slag or metal at room temperature has been the goal of both process metallurgists and melting operators for many years.
There are several rapid methods for estimating various constituents in steel in addition to the conventional chemical methods which are quite fast, but these do not reveal the nature of the slag as a refining agent, which is of primary interest to the steelmaker.
Particle Size of Slag Powder
A large sample of commercial blast furnace slag of intermediate basicity (V-ratio 1.15) was selected for the study. The slag had been put through a jaw crusher until all of it passed through a 20 mesh screen. Five fractions of this crushed material were separated, -20 to +40, -40 to +60, -60 to +100, -100 to +200, and -200 mesh.
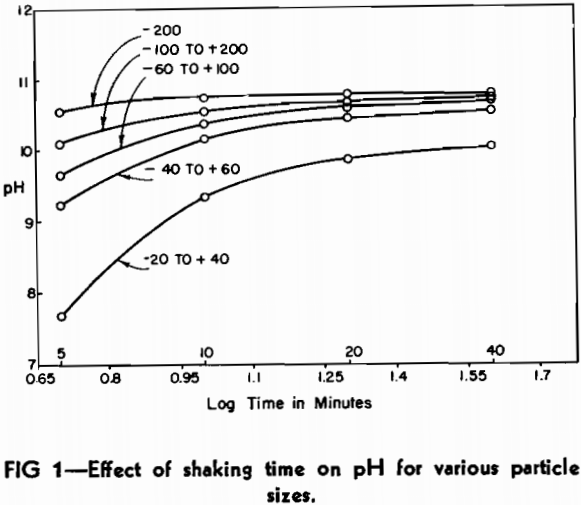
A representative sample of 0.5 g was removed from each fraction and the pH determined using the method of Philbrook. Check pH analyses on the sample fractions varied due to the different amounts of shaking. To eliminate this variable, a mechanical shaker was employed. In order to know the exact time of contact between the slag and water, it was found necessary to filter the extract at the end of the shaking period. Using the mechanical shaker and a filtering apparatus, similar runs were made on the five fractions for contact times of 5, 10, 20, and 40 min. Random checks gave reproducible results within 0.02 pH. The data are plotted in Fig 1.
It can be seen from the plot that each slag fraction is hydrolyzed to an extent that is roughly proportional to the surface area exposed to the water. The (—100 to +200) mesh material changed very little in pH after 10 min. shaking time. The curves are symmetrical and lie in proper relation to one another. The —200 mesh curve appears to be somewhat flatter than the others, but this can be attributed to the portion of very fine material that is not present in the other fractions.
 Effect of Weight of Sample on pH
Effect of Weight of Sample on pH
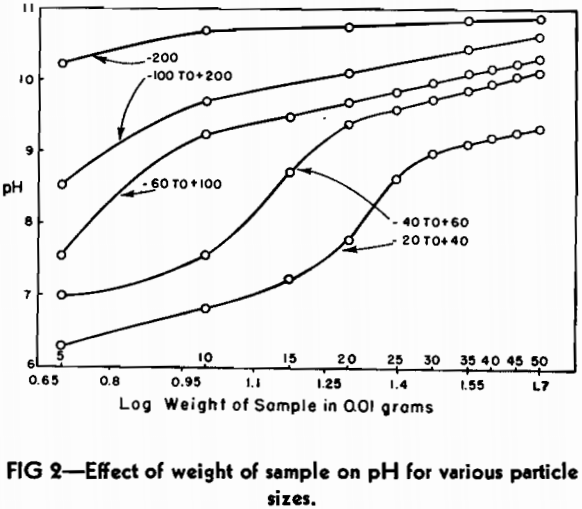
The same commercial blast furnace slag that had been used in the study of particle size was also employed in determining the effect of weight of sample on pH. Portions of the five screen sizes were removed and weighed carefully on an analytical balance. The smallest sample taken was 50 mg and the largest sample was 500 mg.
It can readily be seen from the plot that the curves assume the general characteristics of potentiometric titration curves for acid-base reactions. This type of curve has a straight line portion on either side of the neutralization point. It was considered important that the sample size selected as a basis for a standard should be well within the straight line portion of the curve in order to compare different slags satisfactorily. As a consequence, the weight of sample selected as standard for the rest of the investigation was 0.5 g.
Standard Technique used for pH Measurement
Weigh out a representative 0.5 g sample of slag which has been powdered so that the entire sample will pass through a 100 mesh screen. The sample should be dry and free of contamination. Weighing of each sample is recommended in order to obtain reproducible results; however, an ordinary trip balance may be used satisfactorily.
Place the sample in a clean, dry 250 ml Erlenmeyer flask. Measure with a pipette 100 ml of previously boiled, distilled water and add to the sample in the flask. As the water contacts the slag, note the time of contact. After the pipette drains, stopper the flask tightly and place in a mechanical shaker. This type of agitation prevents caking and agglomeration of the sample on the bottom of the flask.
Remove the flask from the shaker at the end of approximately 9½ min. and pour the suspension onto a glass frit filter as the timer reaches 10 min. This sets the exact time for contact between the water and the undissolved residue remaining.
Correlation of pH of Blast Furnace Type Slags with Composition
Using the standard technique, as described in the previous section, a set of slags submitted in connection with the Doctor’s thesis of Gerald G. Hatch was checked.
The analyses of these slags are reported in the paper of Hatch and Chipman and will not be repeated here. In general, the slag compositions covered a much wider range of values for the four main constituents than is found in the typical commercial blast furnace bisilicate slags, but they are free of MnO. These limits of composition variation are as follows:

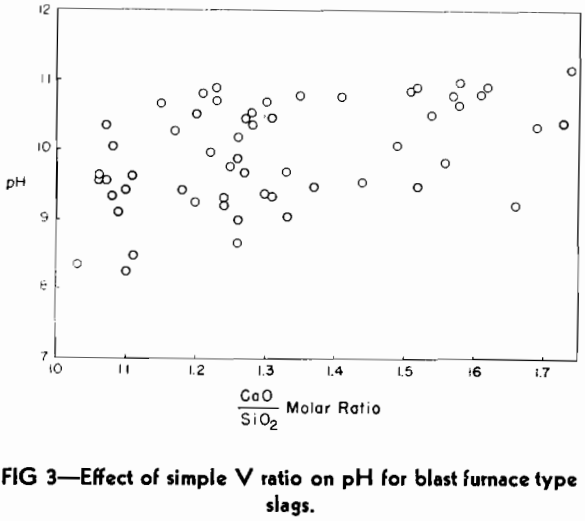
One of the practical limitations of this type of basicity measurement is the variation in the number of millimols of slag in 0.5 g. The replacement of CaO by large amounts of the lighter molecule of MgO will make the number increase, whereas the replacement of SiO2 by substantial amounts of Al2O3 will cause it to decrease.
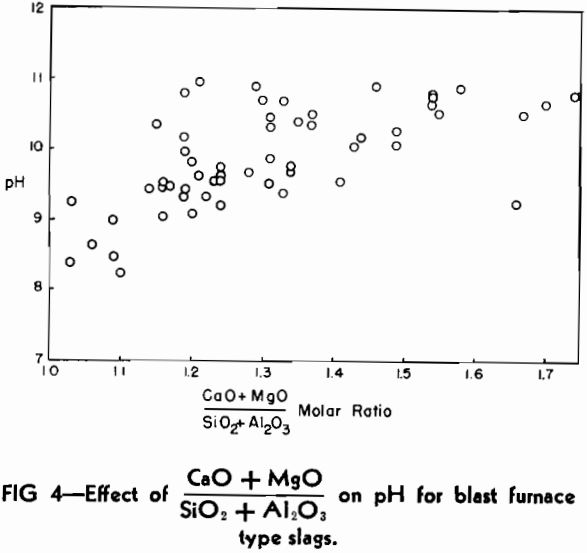
The pH Method of Estimating Slag Basicity
As mentioned previously, the most serious limitation in the use of the pH method as a research tool would be the variation in the number of millimols of slag in a definite weight of sample when composition varies over a wide range of molecular species of widely different molecular weights. In other words, a chemical analysis would still be necessary to check the pH results if it were expected that significant variations might occur in the low molecular weight (MgO) or high molecular weight (Al2O3) oxides from heat to heat.

