Table of Contents
Arsenic, copper, and molybdenum were effectively removed from arsenic-bearing flue dust using a three-stage continuous sulfuric acid leach. Leach conditions to maximize arsenic and copper extraction while minimizing bismuth extraction were determined: 25-wt pct H2SO4, 20- to 25-wt pct solids, a leach temperature of 55° C, and a 30-min residence time. These conditions yielded arsenic, copper, and bismuth extractions of >95, >85, and <25 pct, respectively. Use of bleed electrolyte was shown to be a viable option to reagent-grade sulfuric acid. Bleed solution from an electrolytic refinery was as effective as solutions of reagent-grade acid in the leaching process. The leached residue contained approximately 28 pct Pb with about 5 pct Cu and would be suitable for processing in a lead smelter.
TBP was shown to extract arsenic and molybdenum from pregnant leach liquor without extraction of other metals. Arsenic was selectively stripped with water, and molybdenum was removed with 0.5N ammonia. With the arsenic removed, copper was recovered by cementation.
Environmentally sensitive contaminants such as arsenic, lead, cadmium, and other metals contained in sulfide ores are often concentrated in waste streams such as flue dust, water-treatment sludge, and furnace liners. New and evolving environmental regulations require proper handling and disposal of such wastes and have prompted the development of improved methods for separation, recovery, stabilization, and disposal of metals contained in these materials.
Arsenic poses an environmental problem because of its toxicity (U.S. Department of Health, 1977) and the mobility of dissolved arsenic compounds in groundwater (U.S. Public Health Service, 1987). Arsenic removal and safe disposal are significant problems for some mineral processors. Arsenic stabilization as calcium-iron arsenates (Harris and Honette, 1987; Smyres , 1989) has been recommended for safe disposal or storage, but recent studies suggest the stability of these compounds may be limited (Harris and Monette, 1987; Robbins, 1987). The U.S. Bureau of Mines has researched the processing of several arsenical wastes to recover valuable byproducts and stabilize the arsenic in environmentally acceptable forms (Bloom et al., 1982; Smyres et al ., 1986) or recover it as a product or a concentrated waste material (Madsen and Shirts, 1983).
Recently, the Bureau conducted batch leach tests to investigate the effects of various leach parameters on the removal of arsenic from arsenic-containing flue dusts (Gritton et al ., 1990; Gritton and Gebhardt, 1990). Those bench-scale studios detailed the effects of waste composition, pulp density, leach time, and temperature on the sulfuric-acid leaching of an arsenic-bearing material. Sulfuric acid leaching was used because sulfide mineral processors generally produce sulfuric acid as a byproduct, thus providing a cheap source of raw material.
Sem1contInuous bench-scale sulfuric-acid leaching of an arsenic-bearing waste material is described in this paper. Leach studies investigated the effects or acid strength, pulp density, residence time, and the use of a refinery-bleed solution as the leaching agent on removal of various metal values from the flue dust. The recovery of arsenic and molybdenum from leach solution using tri-n-butyl-phosphate (TBP) was examined with subsequent copper recovery by cementation.
Materials
Smelter Flue Dust
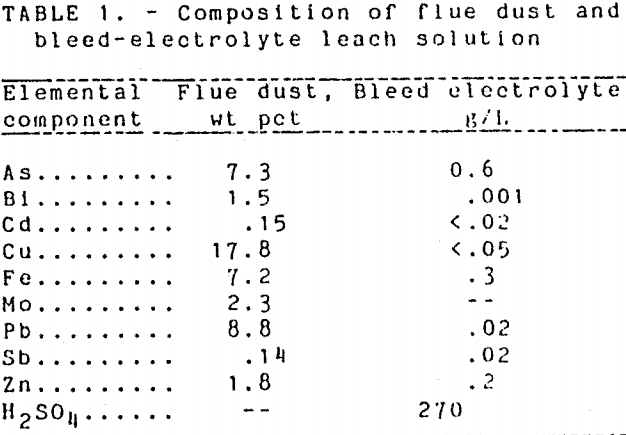
The semi continuous leach studies were carried out using a flue dust of composition similar to those used in previous investigations (Gritton and Gebhardt, 1990). The composition of the flue dust is shown in Table 1. The dust particles averaged less than 10 µm in diameter and were a mixture of metallic copper, as well as copper, iron, and lead sulfates, entrained refractory material, and other oxidized metallics such as As2O3. Characterization of the flue dust, including micrographs of dust samples, was reported previously (Gritton and Gebhardt, 1990).
Bleed Electrolyte
In the electrolytic refining of copper anodes from smelting operations, bleed electrolyte must be removed to control impurity concentrations. The bleed electrolyte is processed for metal recovery or is disposed after neutralization. The composition of refinery bleed electrolyte used in this study is shown in Table 1. This solution contained up to 270 g/l. H2SO4 and was tested as a source of acid for leaching arsenic-containing flue dust.
Continuous Leaching of Flue Dust
Leaching with Sulfuric Acid
Previous research indicated that acid concentration, solids content in the slurry, and residence time most influenced leaching of arsenical wastes (Bloom et al., 1982; Critton et al., 1990; Gritton and Gebhardt, 1990). These parameters were investigated in a continuous leaching system at 55° C with air sparging using conditions listed in Table 2. Air sparging was used to maintain oxidizing conditions; however, flow rates were not monitored. The measured response was the weight percent metal extracted.

A series of 20 tests were performed to investigate the effects of these parameters on metal extractions. Metal extractions were calculated from analyses of the aqueous leach liquors, residue-wash liquors, and solid residues.
Tests were conducted in a three-tier cascading reactor system, shown schematically in Figure 1. The three-tier system was used to narrow the average retention distribution curve. Each reactor had a 400-mL capacity and was equipped with a turbine prop agitator for homogeneous mixing and an air-sparge tube for oxidation. A screw feeder was used to dispense the flue dust to a pulp feeder where dust and acid solution were mixed prior to overflowing into the first reactor. The screw feeder delivered 75 to 400 g/hr dust at a constant rate. The pulp feeder consisted of a 70-mL beaker adapted with a hooded overflow spout to the first reactor. The hooded spout prevented acid fumes from interfering with the screw feeder. Retention time and pulp density were controlled by the adjustment of acid and dust feed rates.
Each test was operated at the given condition for a period equal to 3-5 times the residence time prior to sample collection. This allowed near-equilibrium conditions to be obtained prior to sample collection. The test was then operated for a period equal to the additional residence time during which all streams were collected for analysis. Leach slurries were continuously filtered using a Buchner funnel. Collected residues were repulped in 400 mL of 55° C water to remove soluble copper sulfate or other soluble materials that precipitated during the filtering step.
Metal Extraction Via Leaching
Various metal extractions for specific test conditions are listed in Table 3 and results for specific metals follow.
Arsenic: Arsenic extraction ranged from 84 to 99 pct as shown in Table 3. Extraction increased with increasing acid availability as depleted in Figure 2, where extraction is plotted against the acid-to-dust weight ratio. The dependence of arsenic extraction on the pulp solids and residence time was negligible.
Cadmium and Zinc: Cadmium and zinc extractions wore also dependent on acid availability and are shown in Figure 2. Extractions exceeded 90 pct at higher acid-to-dust ratios.
Copper: Recovery of the copper content, 18 wt pct in the dust, would significantly improve the economics of a flue dust treatment process. Copper extraction ranged from 85 to 92 pct over the range of conditions used. A contour plot depicting copper extraction as a function of solids and acid concentration was generated using response-surface methods (Wadsworth, 1990). Predicted extraction contours for a 30-min residence time are depicted in Figure 3. As indicated by the extraction gradients, copper solubility was most influenced by acid concentration and was sensitive, to a lesser extent, to solids content. Residence time did not significantly affect copper extraction.
Approximately 8 to 10 pct of the copper remained insoluble even with 37 pct H2SO4 in the leach. The insoluble fraction was probably metallic copper; metallic spheres had been identified in previously reported electron micrographs (Gritton and Gebhardt, 1990).
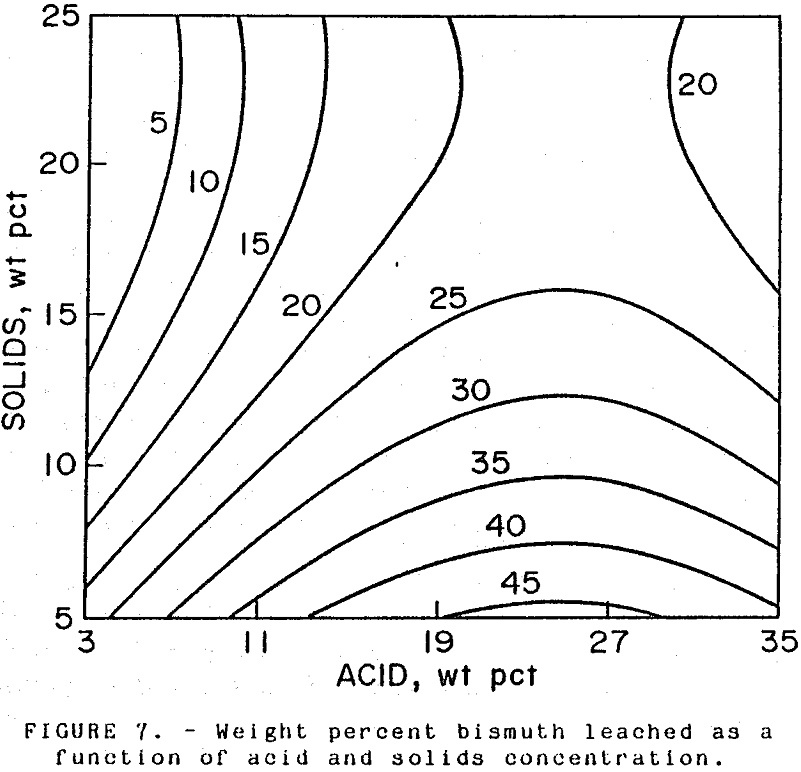
Bismuth: Separation of bismuth from copper is desired because of the potential for bismuth to adversely affect downstream copper-refining operations. Unleached bismuth would remain with the lead-bearing residue and be removed in a lead smelting process (Woodbury, 1985). Bismuth extraction increased with acid availability, as shown in Figure 4, where bismuth extraction is plotted against acid-to-dust ratio. Extractions ranged from 3 to 34 pct. Other leach parameters had little effect on bismuth extraction.

Iron: Acid is consumed in leaching iron which is of no commercial value in this process. The extracted iron would remain with the leach liquor and require subsequent processing in a neutralization plant. The acid consumed by iron dissolution would not significantly affect operation costs if blood electrolyte or other waste streams were used as an acid source. Iron extraction showed a strong dependence on acid availability as seen in Figure 4. Iron extraction ranged from 28 to 61 pct.
Molybdenum: Another valuable metal in the flue dust is molybdenum, and its recovery could help offset processing costs. Residence time had no discernable effect on molybdenum extraction while acid and solid concentrations both appear to affect molybdenum solubility in a complex manner. Correlation of these parameters with extraction was not possible. Extractions ranged from 81 to 97 pct over the range of operating conditions.
Leach Residues: The leach residues contained from 21 to 31 pct Pb and between 1.8 and 6.8 pct Cu. The bismuth content ranged from 2.8 to 1.2 pct and the arsenic content ranged from .34 to 3.2 pct. Lesser amounts of the other assayed metals were also present in addition to sulfate and silica. Such a residue would likely be suitable for processing at a lead smelter (Considine, 1974).
Leaching with Bleed Electrolyte
Bleed electrolyte (diluted and undiluted) was used to leach flue dust in the semi continuous system for comparison with pure sulfuric acid solutions. A series of tests were conducted using 25-wt pct reagent – grade sulfuric acid, bleed electrolyte solution, and a 50:50 mixture of the two. The continuous leach procedure was described previously, and the following conditions were used: 20-wt pct solids, 55° C, and 30-min leach time.
Metal extractions were virtually the same using either bleed electrolyte or reagent-grade acid as shown in Table 1. Therefore, the leach can be accomplished using this waste bleed stream as a source of sulfuric acid.
Optimum Leach Conditions: Maximizing copper and arsenic extractions while minimizing bismuth dissolution were the main objectives in the selection of optimum leach conditions. Since the effect of residence time was negligible in the range considered, a 30-min leach time was selected, as this condition was easily obtained in the bench-scale leaching apparatus. Operating ranges, which limited bismuth extraction to 20 and 25 pct for a 30-min leach, are presented in Figures 5 and 6, respectively. The unshaded areas correspond to arsenic and copper extractions above 95 and 85 pct, respectively.
An acid strength above 15 wt pct is required to maintain the high arsenic and copper extractions. Solids in excess of 12 wt pct limited bismuth extraction to 30 pct or less (see Figure 7), while increasing the solid concentration above 17 wt pct decreased bismuth extraction to 25 pct. The operating conditions needed to hold bismuth extraction below 20 pct would be restricted to a limited range as illustrated in Figure 6.
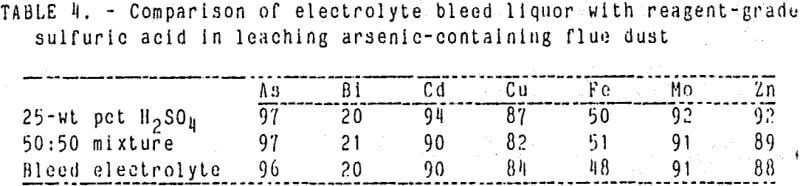
A typical operating range for optimum leach conditions might be 20 to 25-wt pct solids, 25-pct H2SO4 solution, and a 30-min leach time at 55° C. These conditions would yield arsenic and copper extractions of 95 and 85 wt pct, respectively, while limiting bismuth extraction to less than 25 pct. Leach tests using these conditions were conducted to compare the use of reagent-grade acid with the bleed electrolyte solution. Metal extractions were within the above-mentioned limits; thus, the use of bleed electrolyte in the leach is feasible.
Arsenic Removal from Leach Liquors Via Solvent Extraction
The removal of arsenic from the acidic leach liquors was necessary to prevent contamination of byproducts and to eliminate the danger of arsine formation in the cementation recovery of copper. Tri-n-butyl-phosphate (TBP) has been demonstrated effective in extracting arsenic from aqueous solutions at low pH (Kerfoot, 1978). Whether other metals contained in the leach liquor would be coextracted with the arsenic was unknown, and studies were initiated to determine the selectivity of TBP for arsenic over other metallic ions.
Extraction With TBP
Batch tests were conducted in which undiluted TBP was contacted with leach solution generated in the semicontinuous leach circuit. While the composition of solutions used in these screening tests varied slightly, typically the pH was near 1.0, and the composition was approximately, in grams per liter, 12 As, 0.2 Bi, 0.14 Cd, 25 Cu, 2 Fe , 2 Mo, and 1.5 Zn. Contact was made in Mixxor cells with contact times of 2 min. Mixxor cells are designed to maximize phase contact and generally require contact times of less than 2 min to reach equilibrium. Several aqueous-to-organic (A-O) ratios were evaluated for construction of a McCabe-Thlele stripping isotherm. All contacts were made at room temperature.
Only arsenic and molybdenum were extracted by TBP; virtually none of the other metallic ions were detected in the organic solutions after contact with the leach liquor. Results indicated that four theoretical stages would reduce the leach liquor’s arsenic concentration from 12 to 0.5 g/L at an A-O ratio of 1 : 4 as shown by the McCabe-Thiele diagram in Figure 8. Molybdenum extraction would be complete in one theoretical stage.
Selective Stripping of TBP
Arsenic and molybdenum were selectively stripped from the loaded organic in a two-step procedure. Batch stripping tests were conducted by contacting pregnant TBP with water using the same testing procedure outlined for the loading study. Several A-O ratios were evaluated to construct a McCabe-Thiele stripping isotherm. A similar procedure was used to evaluate molybdenum stripping with 0.5N ammonia.
Contacting the loaded organic with water at room temperature stripped only arsenic, while contact with 0.5N ammonia was necessary to strip molybdenum. Three theoretical stages, at an A-O ratio of 1:1, would completely strip arsenic with water and one ammoniacal stage would remove molybdenum.
Arsenic and Molybdenum Products
Research on the recovery of arsenic and molybdenum products from the strip solutions is incomplete at this time, but previous Bureau research has demonstrated the feasibility of recovering arsenic as either As2O3 or arsenic sulfide (Madsen et al., 1983). Molybdenum recovery from the ammoniacal strip liquor as ammonium molybdate would be a viable option (Considine, 1974).
Copper Cementation
Copper was recovered from the solvent-extraction raffinates by cementation on iron. Batch tests were conducted in which a liter of leach solution (arsenic removed via solvent extraction) was contacted with powdered, reagent-grade iron using 75, 100, and 200 pct of the stoichiometric requirement. Tests were conducted under agitation for 1 h at room temperature in an enclosed system. Since the evolution of small amounts of arsine during cementation was possible, an indicating scrubbbing system , utilizing silver- diethyl – dithio-carbonate in pyridine and potassium permanganate, was used to detect and oxidize any evolved arsine (Snell and Snell, 1959). No arsine evolution was observed during the cementation tests.
Copper Recoveries
Copper recoveries and product compositions are summarized in Table 5. Adding the stoichiometric amount of iron cemented only 59 pct of the copper while increasing the addition to 200 pct stoichiometric iron resulted in complete copper precipitation. The product was comprised of 70 pct Cu, 13 pct Fe, and smaller amounts of the other metals.