Table of Contents
The solid amalgam, which is retained in the canvas or wash-leather filters, usually contains from 30 to 45 per cent, of gold and silver, according to the state of division of the gold present in the ore, and also to the degree of care exercised in squeezing out the excess of mercury. For separating the gold from the mercury there are two kinds of retorts in general use—the pot-shaped retort, which is sometimes cast with trunnions to swing on supports, in small mills; and the cylindrical retort, shown in Fig. 39, in larger mills. The pasty amalgam is rolled up into balls or kneaded into cakes, and squeezed into the pot-shaped retort, and often rammed down with a bolt-head, although this course is deprecated by some managers, who prefer to leave the amalgam as spongy and open in texture as possible, believing that a uniform product is thus obtained more rapidly at a lower temperature and without so much loss. In the horizontal retort the amalgam is placed in iron trays divided into compartments by partitions. In either case, the retorted metal is prevented from adhering to the iron, either by laying it on three or four thicknesses of paper, the ashes of which remain beneath the amalgam, or by covering the iron trays with a coating of whitening. The mercury is condensed in cooling tubes passed through water; the loss through volatilisation is usually very small, and may be taken as being about one grain of gold per pound of mercury.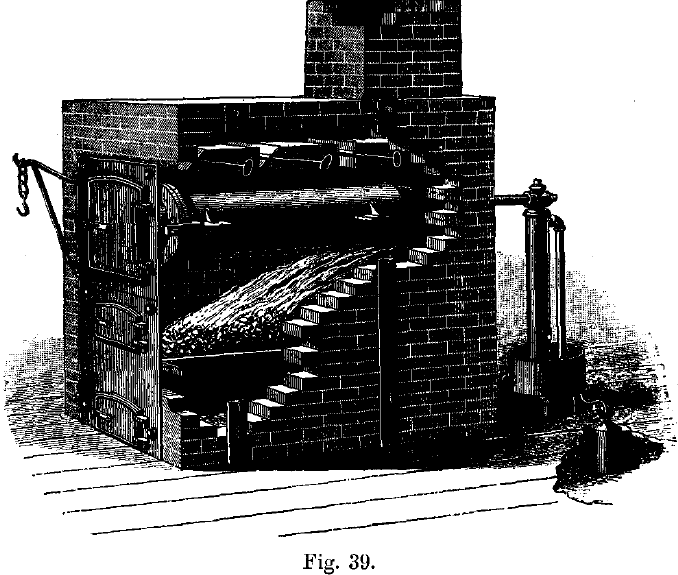
The charge is heated slowly until the boiling point of mercury is reached, when the fire is checked, and the retort kept at an even temperature for three or four hours, or until the bulk of the mercury has been driven off. The retort is then raised gradually to a bright red heat to expel the remainder; after cooling, it is opened, the trays withdrawn, the retorted metal loosened by a chisel, if necessary, and turned out on a table.
In retorting amalgam containing considerable quantities of base materials, there is a danger of the vent being choked up by condensation of solid material. The retort should be so arranged that a rod can be passed through the condensing pipe so as to clear it of obstructions, if necessary. The front of the retort is luted on carefully with chalk or wood-ashes and salt, and firmly clamped so as to be quite tight, otherwise a loss of mercury is incurred. In all retorts the lid is turned and ground so as to fit on perfectly. The condensing pipe should not have an open end dipping freely into water, as in that case a sudden cooling of the retort would cause the water to be sucked in, and an explosion would occur. The open end of the pipe may be enclosed in a bag of sacking or rubber immersed in water, or the pipe may be continued by a piece of sacking which dips under water.
The pot-shaped retort requires no brick fittings, and can be heated over an assay furnace or forge fire, or in a fire built on the ground, when it is placed on a tripod stand. In the latter case the fire is lit at the top and burns slowly downwards. The pot-shaped retort is not filled to more than two-thirds its capacity, and must be heated very gradually at first.
The retorted metal is porous and from 500 to 950 fine in gold, the remainder being in general chiefly silver, with base metals and sulphides in smaller quantities. The gold is melted in crucibles with sand, carbonate of soda, and borax, and suffers a further loss in weight, due to the slagging off of oxides, earthy impurities, &c., and to the volatilisation of a small quantity of mercury, which is obstinately retained until the melting takes place. This amount need not be more than from 0.5 to 1 part per 1,000 of the retorted metal, if the retorting has been carefully performed and finished at a high temperature.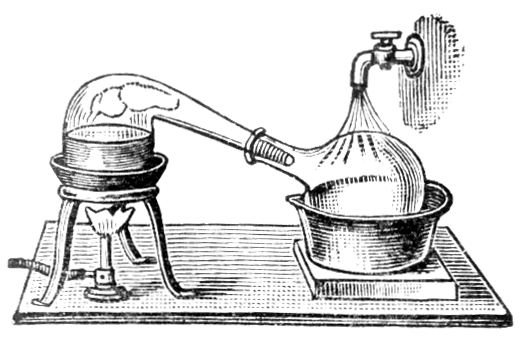
Richards states that if the amount of mercury is to be reduced below 1 or 1½ per cent, in retorting, a white heat must be used, by which the retorts are damaged and soon worn-out. Additions of nitre, corrosive sublimate, &c., are not to be recommended. The melting of bullion is dealt with fully in Chapter xviii. The loss of weight in melting is given by Richards as 1½ per cent, at the Homestake Mill and 7 per cent, in the Caledonia Mill. Part of this loss consists of gold and silver retained by the slag which is either remelted or passed to the stamp mill or clean-up barrel.
Minimize Mercury Losses
The loss of mercury is due to two causes, “ flouring,” or minute mechanical subdivision, due to excessive stamping or grinding, and “ sickening,” or extreme subdivision caused by chemical means. In the latter case, a coating of some impurity is formed over the minute globules of mercury, which are thereby prevented from coalescing, from taking up gold and silver, or from being caught by the plates and wells, as the coating prevents all contact between the mercury and other bodies. The impurity may be an oxide, sulphate, sulphide, or arsenide of some base metal, either originally present in the mercury, or taken up from the ore by it; occasionally the mercury itself may be partly converted into a sulphate or other salt, although this latter condition is not common. The employment of mercury, which contains no base metals dissolved in it, will reduce the loss due to sickening, but such pure mercury is not always obtainable (except by very careful distillation, in which the first and last portions condensed are rejected), and it soon takes up fresh, impurities when used with sulphuretted ores. The base metals usually present in mercury are rapidly oxidised in the air, especially in contact with water; the oxidation is made much more rapid by the presence of any acid in the water, and this acidity (due to the presence of acid sulphates from decomposing pyrites) is rarely quite absent from battery and mine waters. The metallic oxides thus formed are not soluble in mercury, and they float on its surface in the form of little black scales, which soon form a coating. Impure mercury, when used to amalgamate the plates, causes their discoloration by oxidation of the dissolved metals. One of the impurities in mercury most to be feared is lead, as the amalgam of this metal tends to separate out of the bath of mercury in which it is dissolved. According to Prof. J. Cosmo Newbery, it rises to the surface by degrees, taking with it any gold amalgam that may have been formed, and floats as a frothy scum, coating the mercury and preventing any further action by it, whilst it is readily powdered and carried away in suspension by a current of water flowing over it, so that the gold contained in it is lost.
Sickening of the mercury is also promoted by base minerals present in the ore. Most gangues, except heavy spar, hydrous silicates, &c., have no action on the quicksilver; even clean cubical iron pyrites, and other iron and copper pyrites, if they are undecomposed, are harmless, although the materials last named cause sickening when partly decomposed. The other sulphides are all more or less harmful, their action being, however, much less energetic than the compounds of arsenic and antimony. J. Cosmo Newbery conducted a number of experiments in Australia many years ago, to determine the action of some of the base metals on mercury, and found that compounds of arsenic and antimony are particularly harmful, and that if gold containing metallic arsenic is amalgamated, the resulting amalgam is black and powdery, and floats on the mercury, being coated by black metallic arsenic, which separates out and refuses to unite with mercury. Arsenical pyrites seems to act in the same way as metallic arsenic, a large amount of black sickened mercury being produced by it, the action being especially energetic if the pyrites is partly decomposed. The black coating is, in this case, a mixture of pyrites, arsenic, and mercury, in a very finely divided state. Sodium amalgam acts beneficially when arsenic is causing loss of mercury.
Sulphide of antimony breaks the mercury into black powder even more quickly than arsenic, some sulphide of mercury being formed if there is any trituration, whilst the antimony forms an amalgam. The action of sodium amalgam on this mixture is of no avail, as sodium sulphide is formed, more antimony amalgam produced, and sulphuretted hydrogen set free, the results on the amalgamation of the gold being very disastrous. Bismuth sulphide acts similarly, but with less rapidity.
Floured mercury is perfectly white in appearance, like flour, sickened mercury, as already stated, being blackish. If this floured mercury is examined with a lens, it is seen to consist of a number of minute particles—many of them microscopic—each of which is perfectly bright and pure, shining like a mirror. They are prevented from coming into contact and coalescing by being surrounded by films either of air or of some transparent foreign substance. Floured mercury is readily carried away and lost in the tailings, but if passed through and agitated with a large body of clean mercury much of it is at once absorbed in the mass. The loss through flouring is experienced in the milling both of refractory and free-milling ores. The effects of grease and also of talc, serpentine, clay, and other hydrous silicates in subdividing mercury are doubtless due to mechanical action only.
In California the total loss of mercury varies from 1/5 to 1 oz. of mercury per ton of ore crushed, the mean being about ½ oz. per ton. Most of the mercury is lost as such and not in the form of amalgam, as is proved by the fact that where the largest proportion of mercury is fed into the battery the greatest loss takes place but the highest percentage of gold is recovered. Thus, at the North Star and Empire Mills the greatest loss in the State occurs, 1 oz. of mercury being lost per ton, but over 90 per cent, of the gold is extracted. In the Blackhawk Mills, Colorado, where base ores are crushed, containing from 12 to 20 per cent, of pyrites, the loss of mercury is from 1/5 to ½ oz. of mercury per ton. The mills in which the greatest loss of mercury occurs have the deepest discharge, the ore and mercury being in these cases pounded together for a greater length of time before being ejected from the mortar, so that more flouring takes place. At the Ferreira Deep Mill the loss of mercury is given by D. J. Peplar as 0.35 oz. per oz. of gold and 0.16 oz. per ton of ore crushed.
Many suggestions have been made at various times for the reduction of the loss of mercury. The use of certain chemicals in keeping it clean and lively and in neutralising the bad effect of base minerals has already been noticed (see p. 130).
Properties and Purification of Mercury
Pure mercury is unaffected by the air at ordinary temperatures, but is slowly oxidised if heated to about 350° C. It is not acted on by hydrochloric acid, and is almost unaffected by dilute sulphuric acid, but with hot concentrated sulphuric acid it forms HgSO4. Mercury is dissolved even by cold dilute nitric acid, and is rapidly dissolved by hot nitric acid. Pure mercury will roll down an inclined surface without forming a pronounced “tail” and without leaving any streak behind it. If a blackish film is left behind, the mercury requires purification.
When agitated with oil, fats, turpentine, many organic substances, sulphur, &c., mercury is split up into minute globules, not easily re-united. This is known as the “flouring” of mercury. Vegetable or animal oils cause more flouring than mineral oils. Coalescence of floured mercury is effected by the action of certain reducing agents, such as water and sodium, the passage of an electric current, or with some loss by the action of nitric acid.
Types of Amalgam
Mercury forms amalgams directly with gold, silver (more readily if heated), copper, lead, zinc, and bismuth. Amalgams of tin and cadmium are formed directly with great ease. Mercury unites with antimony and arsenic only if heated. Antimony gradually separates from its amalgam as a black powder. Iron amalgam is formed directly only if the iron is finely divided. When iron amalgam is retorted, pyrophoric iron is formed, yielding a somewhat troublesome mixture with gold. Amalgams of nickel, cobalt, manganese, chromium, aluminium, and platinum are not formed directly, but are formed indirectly by electrolysis of their salts with mercury as the negative pole. Amalgams of sodium and potassium are formed directly with the aid of heat. The amalgam Hg12Na2 is solid, containing about 2 per cent, of sodium.
How to Purify Mercury
It is very important to use pure mercury in ordinary amalgamation processes, so as to reduce the losses as far as possible. The purification may be effected by distillation with lime and iron filings. The iron filings decompose sulphides and prevent bumping. An addition of charcoal powder is mentioned by Richards, its use being to prevent the formation of volatile oxides. Lead and zinc pass over in part with the mercury. Zinc, tin, copper, and iron may be removed by shaking with dilute hydrochloric acid. Floating impurities are removed by running the mercury through a glass funnel, regulating the discharge by a finger placed over the stem-hole. If mercury is covered by dilute nitric acid (one part of acid to three parts of water) it is gradually purified, especially if stirred occasionally. Mercurous nitrate is formed and acts on the base metals. A more rapid way of removing base metals is to pass a stream of air through mercury covered with dilute nitric or sulphuric acid. The base metals are rapidly oxidised by the air and dissolved by the nitric acid. This method has been found useful by T. C. Cloud.
