Table of Contents
The copper-silver-mercury orebody of Gortdrum Mines (Ireland) Limited is located 3 miles north of the town of Tipperary in the Republic of Ireland.
The first shipments of concentrates were made to the smelter in Europe in late 1967. In the spring of the following year, word was received from the smelter that these early shipments contained between 1% and 2% Hg, and that the smelter could not accept further shipments of this quality.
Roasting was the only feasible method found for removing the mercury from the concentrate, and it was decided that this should be done in an atmosphere with limited oxygen.
Early Work
One of the first requirements was to establish the extent of the problem. An initial difficulty was with analyses. It was obviously desirable to know how much mercury was in the ore, and, since arsenic and antimony were important penalty elements in the copper concentrate, it was also important to be able to analyse the ore for these elements.
Reasonably reliable techniques were soon developed for the analysis of concentrates, although doubts about the absolute accuracy persisted for a considerable time. The methods adopted were atomic absorption techniques for mercury and antimony, and a distillation technique for arsenic.
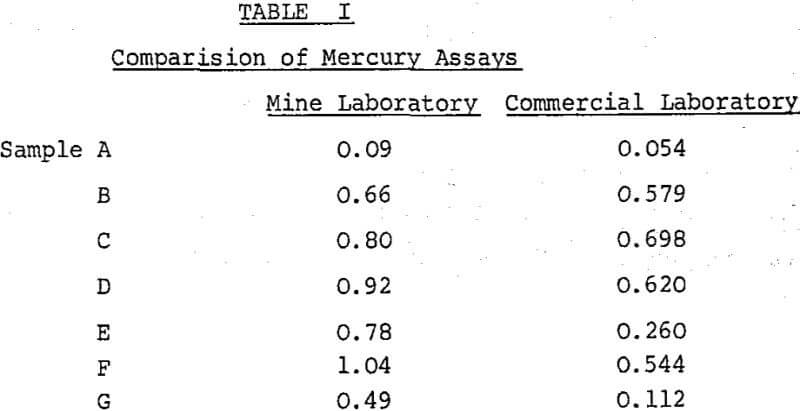
It seems incredible that such discrepancies could arise on what should be a routine analysis. Point is given to the observation that any assay is only an estimate, with variable accuracy and precision. Too often, assays are regarded as absolute.
Types of Mineralisation
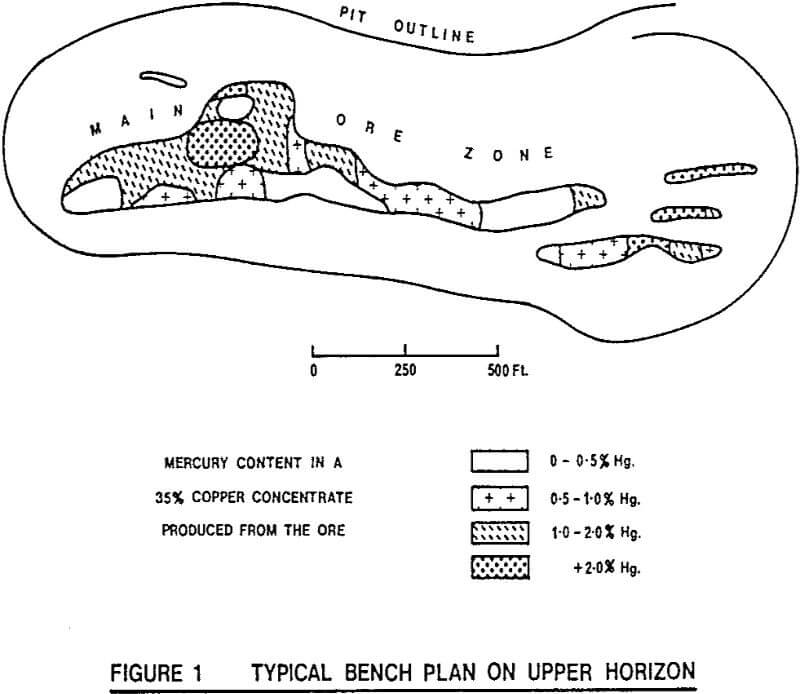
The Gortdrum orebody occurs in Carboniferous limestone, where it is faulted up against a Devonian sandstone. The gangue is mostly limestone with a little quartz and barite.
The sulphide mineralisation consists almost entirely of a variety of copper minerals, and is noteworthy for the virtual absence of other sulphide minerals. The principal copper minerals are bornite, chalcopyrite, tennantite-tetrahedrite and chalcocite, with minor covellite and native copper.
The tennantite-tetrahedrite series of minerals also has the feature that some of the copper atoms in the crystal lattice can be replaced by other heavy metals, such as zinc and mercury. At Gortdrum, electron micro probe analysis has shown that the tennantite-tetrahedrite mineral can contain up to 5% Hg, as well as about 1% Zn.
Selective Mining
The variable nature of the mineralisation throughout the orebody allowed for the possibility of selective mining.
The possibilities for selective mining are apparent and selective mining has been a feature of the mining operation at Gortdrum until the present time. Even with the mercury plant operating to remove mercury from the concentrate, there has been a distinct need for careful grade control and scheduled mining because of the requirement to maintain the arsenic and antimony assays of concentrate shipments below rigidly specified limits.
Selective mining requires careful scheduling of the mining operation to ensure that the ore of the requisite grade is available at the requisite time. But advance scheduling can only be done in a limited amount of detail, and careful grade control at the time of mining is also necessary to ensure that the ore fed to the mill will produce concentrates of the desired arsenic and antimony content.
From the very beginning, it was apparent that roasting was one way of removing mercury from the concentrates, but other alternatives were considered. The possibility of differential flotation was investigated with the hope that one copper mineral could be separated from another, so that a saleable product could be produced on the one hand, and a product in which the impurities were concentrated on the other hand. In the event, this could not be done.
The batch scale tests could be only partially indicative of the commercial process, so arrangements were made to set up a pilot plant test programme including mercury condensation. The commercial furnace suppliers, Simon Carves Limited, were contracted to build the pilot plant and conduct the testwork in Manchester, England, under Bechtel and Gortdrum’s supervision.
Choice of Process Parameters
The roasting process at Gortdrum is unconventional for mercury processing in that it is conducted in an atmosphere with limited oxygen content i.e., a neutral atmosphere. This particular decision was taken for a number of reasons.
- As far as mercury removal from the concentrate was concerned, the process could be conducted in an oxidising, a neutral, or a reducing atmosphere.
- In an oxidising atmosphere, all of the sulphur in the concentrate would report as sulphur dioxide gas which would have to be removed from the gas stream before it was discharged to atmosphere. By using a neutral atmosphere this problem was avoided.
- Under oxidising conditions, much of the copper was sulphated. It was felt that such a product would be hazardous to ship in bulk in steel ships because of the danger of corrosive attack on steel by soluble copper salts.
- Copper smelters, at that time, preferred sulphide concentrates.
- The particular smelter to which most of the concentrates were to be sold used the Momoda method of feeding its blast furnace. The Momoda method requires that the concentrates, when moistened and pug milled, have a suitable consistency, similar to putty..
Description of Commercial Plant
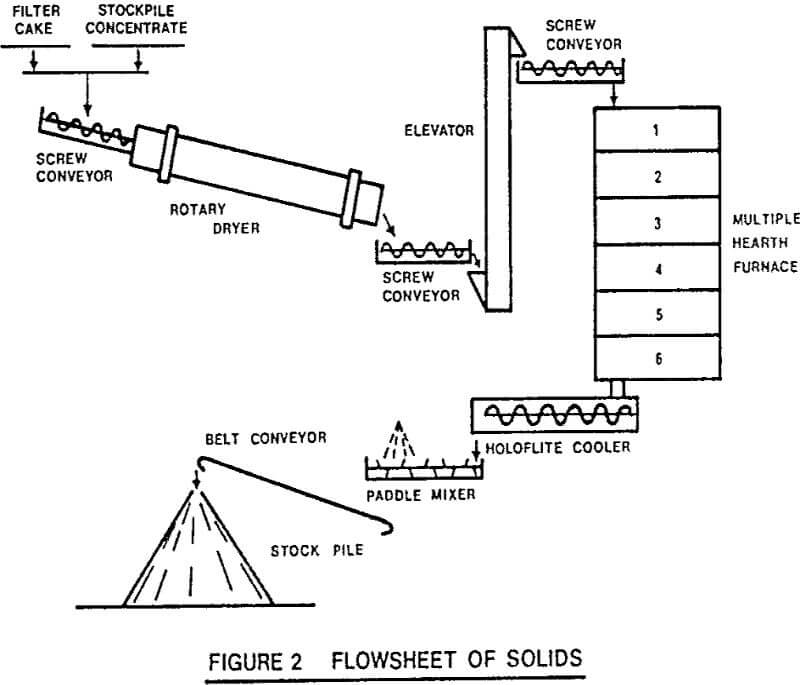
The plant operates to produce 1000 tons per month of roasted concentrate. The feed to the plant consists of either filter cake or stock piled concentrate, or both. This material is dried to 5% moisture in the 4 ft. x 25 ft. rotary dryer. The rotary dryer was included in the plant mainly because it had already been bought when the plant was designed. It also allows the possibility of selectively mined low mercury concentrates being dried for shipment without having to pass through the roasting furnace. The partially dried concentrate passes via screw conveyors and bucket elevators to the top of the multiple hearth furnace.
The furnace is 13 ft. 6 ins. diameter and contains 6 hearths. The steel shell was made big enough so that another 4 hearths could be added below the present 6 hearths. This happened because the furnace was sized and ordered before testwork was completed, and it was felt that the possibility of adding 4 hearths would be wise insurance. In the event, they were never needed.
The calcined concentrate has to be cooled out of contact with the air. This is accomplished in a sealed, water cooled Holoflite screw conveyor, having twin flights 16 ins. diameter by 16 ft. long.
For shipping purposes, water is added to the cooled concentrate to 8% moisture in a paddle conveyor and the moistened concentrate is stock piled for shipment.
The roasting section is operated counter-current with the burner situated on hearth 6 and the mercury laden gases discharging from hearth 3. A cyclone and an electrostatic precipitator are used to remove dust from the gas, the dust being recycled to the furnace feed. The cleaned gases then pass via the hot gas fan to the condensers.
Process Data
It should be emphasized that these temperatures are gas phase temperatures and that the solid materials are at a different temperature, usually lower. Moreover, even on any one hearth, the gas phase temperature will vary depending on where on the hearth it is measured. The temperatures quoted therefore are typical, useful for control purposes, and indicative of the process temperature, but lack any real, precise, meaning.
In the drying section of the furnace the temperature of hearth 2 is maintained at below 200°. At higher temperatures there is a risk of the dust in the bag filter catching fire. With normal low oxygen contents this should never happen, but it has happened that high temperature occurred when the oxygen content was sufficient to allow the dust to ignite. When this happens the glass fibre bags arc destroyed because the temperature then exceeds the limit of 260°c. which the bags can withstand.
Temperature control is dependent upon atmosphere control. If the combustion gas plus quench gas entering the furnace is low in oxygen, as it should be, then virtually all the heat to the furnace has to be supplied by combustion of the oil in the burner. But any oxygen entering the furnace will react with the concentrate to form sulphur dioxide with production of heat.
Start-Up Problems
The commercial plant had a plentitude of teething problems. To some extent this was to be expected since no other directly comparable plant existed in the world, which could be used for guidance, and because of the relatively short time available for testwork, design and engineering. In the design, decisions had to be taken based on insufficient data and experience.
However, other problems should have been avoidable in view of the combined experience of the mine personnel, the engineering consultants and the various manufacturers. It is with the hope that our experience will help others that we discuss our difficulties.
The process itself worked as anticipated from the very beginning as far as removing mercury from the concentrate was concerned, but the problems arose from the equipment within which the process took place. Major difficulties arose in the areas of materials handling, cooling of the calcined concentrate, and with oxygen control.
In order to prevent spontaneous combustion it is necessary to cool the calcined concentrate out of contact with air. The original cooler was a tube cooler supplied by the furnace manufacturer. This consisted of 357 steel tubes each 1¼ inches inside diameter. A gate mechanism at the bottom controlled the discharge from the tubes. In normal operation, the tubes were maintained full of concentrate which was allowed to form a pile on top of the tubes.
Soot Handling
The product from mercury plant condensers is commonly known as soot. The mercury is present as finely divided globules of metallic mercury. The arsenic and antimony are mostly present as the trioxides evolved as by products of the roasting reaction. The copper arises from concentrate dust which has been mechanically carried over into the condensers. The soot is collected under water in the condenser thickener and discharged from the collecting pots as a thick slurry.
At Gortdrum, it was hoped that hoeing would extract 50% to 75% of the mercury. The balance was to be obtained by retorting the hoed residue. This was done in a conventional muffle furnace, externally heated, in which the hoed soot was placed in pans. The liquid mercury was collected in a simple watercooled pipe condenser. This system did not work well. At best, hoeing liberated about half of the mercury in the soot and, at worst, no mercury at all was liberated. Thus the retorting operation was being expected to handle 50%, to 100% of the mercury instead of 25% to 50% as anticipated.
Metallurgical Balance
For the first year and a half of operation, there seemed to be large discrepancies in the metallurgical accounting for mercury, with an apparent large loss of mercury. While no significant physical losses of mercury could be detected, the apparent losses were of great concern, particularly because of the possibility of theft. Mercury is a high value product, which can be readily concealed in a fairly small volume, and which can be quite readily disposed off, and theft is an ever present possibility.
At first, the irregular operation of the plant made any realistic accounting impossible. Then, the necessity of stockpiling hoed soot exacerbated the situation. Hoed soot is almost impossible to sample and assay correctly because of the irregular way the globules of mercury are distributed throughout it. The situation is analogous to sampling high grade gold ore, which is notoriously difficult. Since the hoed soot was a high grade product, assaying about 20% Hg, and represented, for a period, about 50% of the mercury removed from the concentrate, any discrepancies in the figures for hoed soot meant substantial discrepancies in the overall figures.