Table of Contents
Three types of melting furnaces are in general use, all oil-fired for the Melting of Gold Precipitate. For smaller plants treating gold ores, the tilting furnace with removable graphite crucible is usually preferred. Clay liners are quite generally used to prolong the life of the pot.
In larger plants, particularly those treating silver ores, tilting furnaces of the reverberatory type are most satisfactory. Such furnaces are provided with a molded hearth of suitable refractory and may be either single or double, depending on the amount of precipitate to be handled.
In the largest mills treating either gold or silver ores, stationary reverberatory furnaces are used. These may be built to operate as a conventional reverberatory furnace, with the usual fusion hearth of firebrick or other suitable refractor or may be used as reverberatory pot fusion furnaces, with lined graphite pots. This is the furnace generally used in the large mills on the Rand.
Precipitate containing up to 30 to 40 per cent moisture may be fluxed and melted without drying, or driers, either steam or electric, may be used to reduce the moisture to from 15 to 20 per cent before adding flux and charging to the furnace. Where filter presses are used, blowing with compressed air yields a suitable product for melting without further drying.
Fluxes used in melting cyanide precipitate vary somewhat in different parts of the world; the following mixtures are typical, but in starting a new plant trial fusions should be made in each case to determine the most suitable mixture.
For melting raw precipitate from clean gold solutions the following charge will usually give rapid fusions and clean, fluid slags.
- 75 lb. precipitate (15 to 20 per cent moisture).
- 50 lb. borax.
- 45 lb. manganese dioxide.
- 25 lb. silica sand.
Note: Soda bicarbonate or fluorspar may be substituted for part of the borax, and the manganese may be replaced by niter.
Silver precipitate, containing 80 per cent silver or better, is readily melted with the following charge:
- 100 lb. precipitate (15 to 20 per cent moisture).
- 5 to 10 lb. borax.
- 3 to 5 lb. soda bicarbonate.
- 3 to 5 lb. silica sand.
For melting calcined gold precipitates the following charge is used in many plants:
- 100 lb. dry calcine.
- 25 to 40 lb. silica sand.
- 40 to 60 lb. borax.
- 10 lb. soda ash.
- 5 lb. fluorspar.
Note: Five to 15 lb. manganese dioxide may be added in some cases.
Example Treatment of Gold Precipitate
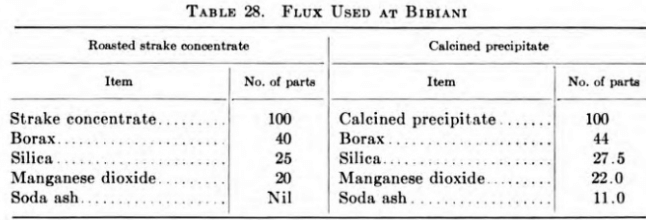
Smelting of both the calcined precipitate and tabled strake concentrate is carried out by the direct crucible method. For plant-control purposes the two plant products are smelted separately in No. 150 Morgan salamander crucibles fitted with fire-clay liners, and the resultant bullion is collected, remelted in No. 60 Morgan salamander crucibles, and cast into bars. The fineness of the bullion obtained is:
Corduroy strake bullion……………………………………………….910 fine gold 995 Dore
Cyanide precipitate…………………………………………………….890
The two products are fluxed as shown in Table 28.
The smelting is carried out in oil-fired furnaces that were fabricated at Bibiani and are a modification of those in use at the Ashanti Goldfields Corporation, Obuasi, Gold Coast. Crucible life averages 50 pours, while that of the fire-clay liners is 8 pours. When new fire-clay liners are fitted to the crucibles, the space between the two is carefully filled and rammed with dry-ground plumbago obtained from old discarded crucibles. All worn-out fire-clay liners are broken up and ground to pass an 1/8-in. mesh screen. Any large prills of gold found are collected and join the smelt, while the fines are sent for retreatment with. the slags.
Treatment of Slags. Approximately 350 lb. of slag is produced monthly, and at the end of each monthly cleanup, this is broken down to 1 in. in size, ground to approximately 100 mesh, and passed over the concentrating tables used for dressing the strake concentrate.
Flux Recipes
The fine free gold concentrate from the slags is de-ironed by a magnet, fluxed, and smelted with the cyanide precipitate. The slag tailing gravitates to a settling sump, is allowed to settle, and the water run off. This final product is then dried, sampled, assayed, and shipped to England for treatment by the Tavener process. Slags shipped range from 5 to 10 fine oz. gold per month.
Other products—such as calciner tray drippings, and floor sweepings— are fluxed and smelted separately.
All furnaces are hooded, and ducts are led off to a bag-chamber dust extractor. This is cleaned out every 3 or 4 months, and the dust smelted separately.
Treatment & Melting of Gold Precipitate
The precipitate recovered by the pilot unit was low grade, averaging 35 to 50 per cent copper and less than 2 per cent gold. As the amount of this precipitate was small, the necessity of refining it was avoided by charging it into the smelter anode furnace. The same procedure was followed during the first year the cyanide plant was operated, but on account of the larger amount of precipitate involved, it became apparent that, in order to account for the gold satisfactorily, some other method of treatment would have to be developed.
Removal of the base metals with acid and melting of the residue to gold bullion was tried on a small scale. Best results were obtained when a leaching period of 5 hr. was employed and when the equivalent of 2 to 3 lb. sulphuric acid per ton of precipitate was used at a dilution of 1 to 1 and a temperature of 100°C., with periodic additions of manganese dioxide totaling not more than 0.2 lb. per ton of precipitate.
This procedure gave base-metal eliminations as follows (in per cent): copper, 96; zinc, 99.9; lead, 17.9; and iron, 66.6; and it left a residue that was 13 per cent of the original weight of the precipitate. From this residue, gold of a high degree of fineness could be produced. The method in detail was, however, quite long and promised to be expensive.
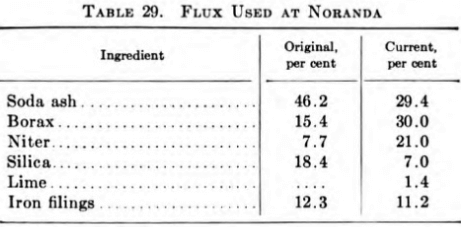
It was decided, therefore, that the best procedure would be to melt the precipitate and produce a copper-gold bullion which could be sampled without difficulty and then be charged into the smelter anode furnace. Melting tests with this object in view were then carried out in sillimanite- lined crucibles. These tests showed that the flux required a high soda- ash content to ensure fluidity of the slag and that sufficient metallic iron had to be used to keep the copper reduced—otherwise the slag carried appreciable amounts of copper and precious metals. They also showed that best results were obtained with approximately 2 lb. of flux per pound of precipitate.
Existing practice is to melt the precipitate to bullion once each month in an oil-fired reverberatory furnace having a capacity of 2000 lb. per 24 hr. The bullion then is remelted in a No. 275 crucible furnace and cast into slabs 15 in. wide, 27 in. long, and weighing 160 lb. After sampling by drilling, these slabs are charged into the smelter anode furnace. The slag is crushed, sampled, and also sent to the smelter, where it is charged into the converters.
The composition of the original flux and of the one currently in use is given in Table 29.
Treatment of Hollinger Gold Precipitate
Here described is the treatment of Hollinger precipitate for the production of fine gold bars and the recovery of silver. At this date (1947) the process is still being used successfully, with some alterations in method from the original, the principal one being the recovery of silver by the Thum process.
There are three main steps in the system:
- the treatment with muriatic acid to remove all the soluble base materials;
- boiling with sulphuric acid, similar but with the necessary modifications because of its nature to the usual acid-parting process, this being possible, since, although the ratio of gold to silver would ordinarily prevent this, the two precious metals exist together not as an alloy but as a finely divided mixture;
- final washing, drying, and melting of the gold residue, in which sodium acetate is used to remove the last traces of lead, and the recovery of the silver from the parting acid.
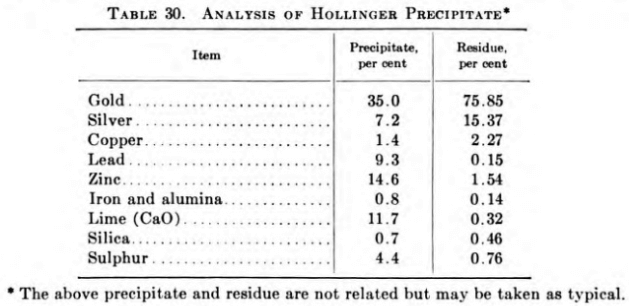
The minimum fineness permitted for the gold is 995, but it is not uncommon for its fineness to be 998.
Silver is precipitated as chloride, filtered, and reduced to metal, using scrap steel from discarded boiling pans; dried; melted; and cast into anode plates for electrolysis. The final bar silver, which is practically free from gold, is approximately 999 fine.
All slags, old crucibles, floor sweepings, and anything that may or does contain gold values, together with high-grade or specimen ore from the mine, are smelted in a cupola furnace to produce a lead bullion that is cupelled, and the resulting gold bullion is added at the melting of silver for anode plates.
In cases where precipitate from outside sources is handled and it is not convenient to treat this for fine-gold production, the muriatic acid step is successfully used, as the residue obtained by this means contains but a minimum of impurities and therefore, after calcination, it is very easily smelted in a small furnace to produce a gold bullion of excellent quality for shipment.
