For melting gold, a strong fire is necessary, and the operation is generally performed in a forge or furnace. The following is a description of a new form of furnace, invented by the author, and which is admirably calculated for melting in crucibles, for the distillation of mercury, for cupellation, and for every other chemical operation requiring the use of a furnace :
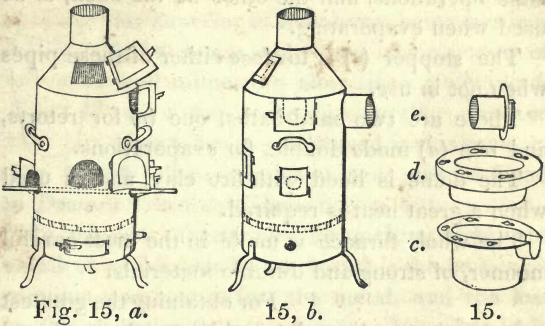
(Fig. 15,) front view, (a.) Side view, (b.) Sand-bath for retorts, (c.) Sand-bath for evaporating, (d.) Stopper for pipe, (e.)
This furnace is 14 inches high, (not including the dome,) and 7 inches diameter. It is made of strong plate iron, and lined with the most refractory fire clay. It has six doors; one in the dome for feeding with coal in crucible operations, one in the middle of the front for feeding, while distilling or evaporating, and for a muffle in cupel operations, and one at the bottom for air ; one door on each side, for iron or porcelain tubes, or for holding an iron bar to support the end of a muffle, and one door in the side at top, for the neck of a retort or sand-bath. There is a small pipe at the bottom, to attach a bellows, and thus convert this into a blast furnace.
There are two pipes for connecting with the flue of the laboratory, one at the top, to be used in crucible operations, and the other at the back, to be used when evaporating.
The stopper (e) is to close either of these pipes when not in use.
There are two sand-baths, one (c) for retorts, and one (d) made double, for evaporations.
The dome is lined with fire clay, and is used when a great heat is required.
The whole furnace is made in the most careful manner, of strong and durable materials.
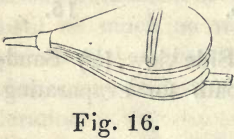 For obtaining the greatest heat which can be produced by the above furnace, a double bellows (Fig. 16) should be connected with the small pipe at the bottom of the furnace, by means of a bent iron pipe, formed so as to raise the bellows a few feet from the floor.
For obtaining the greatest heat which can be produced by the above furnace, a double bellows (Fig. 16) should be connected with the small pipe at the bottom of the furnace, by means of a bent iron pipe, formed so as to raise the bellows a few feet from the floor.
For melting gold, and other metals, good crucibles are required. Of these there are different kinds ; those most generally used for metals are the Hessian. (Figs. 17 and 18.) These crucibles are imported in nests, and may be had of different sizes, in shape either round or triangular. They are generally without covers, and as a substitute for these, a smaller crucible inserted with in a larger one may be used.
 The French crucibles, (Fig. 19,) manufactured by M. Beaufaye, in Paris, and used by the
The French crucibles, (Fig. 19,) manufactured by M. Beaufaye, in Paris, and used by the French chemists and refiners instead of the Hessian, are far superior to the above, being particularly remarkable from their power of well supporting alterations of temperature ; but unfortunately they cost too high to be generally used in this country. A full description of them may be found in Dumas’s Traite de Chimic, t. 2, p. G91.
French chemists and refiners instead of the Hessian, are far superior to the above, being particularly remarkable from their power of well supporting alterations of temperature ; but unfortunately they cost too high to be generally used in this country. A full description of them may be found in Dumas’s Traite de Chimic, t. 2, p. G91.
In the use of crucibles for melting gold, the smallest which can be employed is the best, as it requires less heat to fuse the metal, and the loss in the operation is not so great; but when gold exists in mixture with earthy or stony particles, these ore to be powdered and fused with a flux, and in this case a much larger crucible is required, on account of the evolution of carbonic acid, or swelling of the mass.
The use of the flux is to dissolve the stony particles, forming with them a glass or slag. It also cleans the surface of the particles of metal when heated with it, so as to cause them to unite and fuse into a globule or button, at the bottom of the crucible.
Fluxes of different kinds are used according to the purpose required, but for most all crucible operations with gold and silver, a mixture of one part of the ore with three parts of a flux composed of equal parts of carbonate of potash and of nitre, will answer an excellent purpose. The carbonate of potash in this flux dissolves the silica, &c., in the ore, and cleans the surface of the metal, while the nitre destroys dirt and other organic matters, and at the same time oxydizes any copper which may be present, causing it to become dissolved in the flux.
When gold or silver free from gaugue is to be melted, they require no other flux than a little borax to cause the particles to unite in a button, its use being exactly analogous to that of rosin when used by the tinsmith in soldering.
An important consideration in crucible operations is the choice of fuel. In England, coke is generally used, either alone or mixed with charcoal ; but in America it is difficult to obtain coke, and consequently we are obliged to use anthracite or charcoal. No better fuel than charcoal would be needed if it did not burn away so fast; this is its only disadvantage, as it burns freely, without any clinker, and does not fuse together; but the softer kinds of anthracite, such as peach orchard coal, fuse at a high heat, stick to the crucible, and form large quantities of clinker in the furnace, which makes it very unpleasant to use. The best kind of anthracite is the Lehigh coal. This does not ignite quite so readily as the softer kinds, but it contains less earthy matters, and consequently does not fuse in the furnace or form much clinker.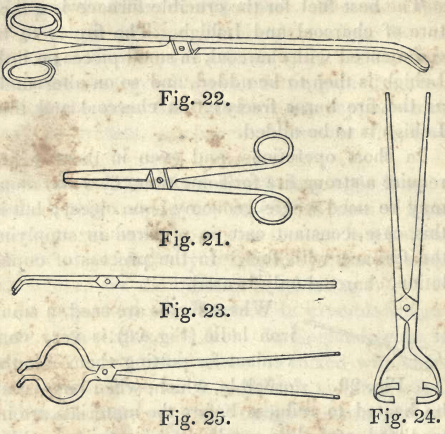
The best fuel for the crucible furnace is a mixture of charcoal and Lehigh. The fire is to be commenced with charcoal, in small pieces; a little Lehigh is then to be added, and so on alternately as the fire burns freely; first charcoal and then Lehigh is to be added.
In short operations, and even in those which require a strong fire for some time, charcoal alone may be used where economy is no object; but in this case, constant care is required in supplying the furnace with fuel. In the process of cupellation, charcoal only is used.
Where fluxes are used, a small iron ladle (Fig. 20) is very convenient for putting them into the crucible, which, when large, may be heated to redness before the materials are introduced, and thus cracks may be discovered before a loss of substance has taken place.
Suitable tongs are indispensable in these operations. The smallest, (Fig. 21,) 8 inches long, for very small crucibles; (Fig. 22,) 16 inches long, made light, and used principally for charcoal; (Fig. 23,) 2 feet long, is the best kind for general operations. A moderate sized crucible, if not too heavy, may be lifted out of the furnace, and the contents poured out with these tongs; but if the crucible is heavy, a piece might be broken out of the side, and the contents lost. To avoid this, tongs are made of the form of Fig. 24 ; these are two feet long, and made so as to clasp round a crucible to take it out of the furnace. Those represented in Fig. 25, are then used to pour out its contents. It is therefore almost absolutely necessary to be provided with a pair of each kind of tongs in order to suit all contingencies.
Amalgamation
The process of amalgamation is used to separate the noble metals from the ore or matrix, when they exist in such small particles as to be invisible, and consequently cannot be picked out by hand.
On the large scale, the ores are ground and amalgamated in mills constructed for the purpose, and composed of two stones. The lower one is stationary, and enclosed in a rim or case, while the upper revolves by the aid of water or steam power. The ore is first stamped, then ground to powder between the stones, and subsequently mixed with metallic mercury, which forms an amalgam with the gold.
It is hardly probable that the readers of this little work will operate so extensively as to require the use of steam or water power. I shall therefore describe in detail the whole process of separating gold by amalgamation, but confine myself to the use of such apparatus and means as can be readily obtained without a great outlay of capital.
Gold often exists in veins, and, when mined, is mixed more or less with stony gaugue. In this case the ore is first to be stamped in an iron mortar, (Fig. 11,) and then reduced to powder by pulverizing in one of porcelain. Fig. 12. A small quantity of metallic mercury 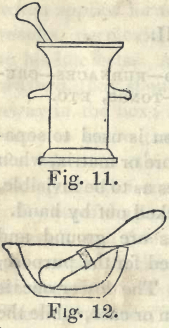 is then poured into the mortar, and the mixture ground together until the gold is dissolved, which is judged of as follows: By adding at first but a very small quantity of mercury, it will soon combine with the gold when ground as directed, and form a solid amalgam. A little more mercury is then added, the grinding continued, and so on repeatedly till the mercury no longer becomes solid by further grinding. Water is then added, and mixed well with the contents of the mortar to wash out the dirt, which is separated by decanting off the water. After a few washings in this way the residue will become clean, and is then to be wiped dry with a cloth or sponge. A piece of chamois leather is now to be spread out in a basin or bowl, and the amalgam of mercury and gold poured into it. The corners and edges of the leather are then to be gathered up, so as to form a bag, with the amalgam at the bottom ; it is tied tightly with a stout string, and the contents squeezed as strongly as possible, by pressing and twisting the bag, by which operation a fluid portion of mercury containing a little gold is separated by passing through the pores of the skin. This fluid portion is preserved by itself, and is to be used in the commencement of another operation on a fresh portion of ore.
is then poured into the mortar, and the mixture ground together until the gold is dissolved, which is judged of as follows: By adding at first but a very small quantity of mercury, it will soon combine with the gold when ground as directed, and form a solid amalgam. A little more mercury is then added, the grinding continued, and so on repeatedly till the mercury no longer becomes solid by further grinding. Water is then added, and mixed well with the contents of the mortar to wash out the dirt, which is separated by decanting off the water. After a few washings in this way the residue will become clean, and is then to be wiped dry with a cloth or sponge. A piece of chamois leather is now to be spread out in a basin or bowl, and the amalgam of mercury and gold poured into it. The corners and edges of the leather are then to be gathered up, so as to form a bag, with the amalgam at the bottom ; it is tied tightly with a stout string, and the contents squeezed as strongly as possible, by pressing and twisting the bag, by which operation a fluid portion of mercury containing a little gold is separated by passing through the pores of the skin. This fluid portion is preserved by itself, and is to be used in the commencement of another operation on a fresh portion of ore.
A solid amalgam of mercury and gold remains in the bag, which is to be taken out and placed in the iron retort. Fig. 13. A little lute, made by mixing powdered pipe clay into a paste with water, is put on the rim of the retort, and after putting on the cover, it is screwed down tightly by means of the clamp. The retort is then placed in a furnace, and heated slowly, at first, to expel moisture, then gradually raised to a low red heat. The end of the retort is meantime placed in a small India rubber bag resting in a vessel filled with cold water, to condense the mercury which distils over and is collected in the bag.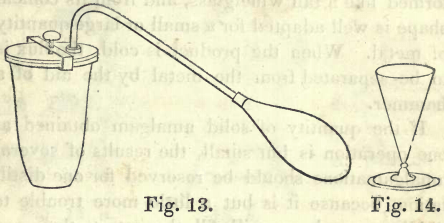
When no more mercury distils over, the operation is finished. The gold remaining in the crucible is of a dull colour, and in a porous mass. It is to be transferred to a small crucible, mixed with a little borax, and melted in the furnace by a strong fire urged with a double bellows. The fused contents of the crucible are then poured into a conical ingot mould. Fig. 14. This mould is formed like a tall wine glass, and from its conical shape is well adapted for a small or large quantity of metal. When the product is cold, the flux is to be separated from the metal by the aid of a hammer.
If the quantity of solid amalgam obtained at one operation is but small, the results of several amalgamations should be reserved for one distillation, because it is but a little more trouble to distil as much as will fill the retort, than one quarter of that quantity ; and a smaller proportion of mercury is lost, which is an important consideration.
The gold obtained in the above process is more or less pure, according to the quality of the ore. It almost always contains a portion of silver, the amount of which, and the standard of fineness of the gold, is to be ascertained by the process of assaying, which will be described in the fourth chapter, together with the process of refining gold.
