Table of Contents
The conversion of coal into a high-Btu gas as a means of augmenting existing supplies of natural gas has been the subject of much research. The Bureau of Mines has played an active role in this area and has developed the Synthane process. The Synthane process consists of reacting coal, steam, and oxygen in a fluid-bed gasifier to produce a raw gas, which is passed through a series of cleanup and conversion operations to produce a clean, high-Btu, synthetic natural gas. The final operation of this conversion process is methanation, a highly exothermic reaction in which carbon monoxide and hydrogen are converted to methane and water.
An essential element for methanation is a catalyst. The catalyst chosen for a demonstration plant of the Synthane process is Raney nickel, a relatively low-cost, highly active methanation catalyst. Raney nickel is an alloy containing 42 pct Ni-58 pct Al developed by Murray Raney in the 1920’s.
The catalyst is available commercially in prealloyed powder form, probably produced by melting and crashing, but specific preparation details are proprietary.
The Synthane demonstration plant is interested in testing the catalyst in forms other than powder. A review of the literature indicated that considerable research has been conducted on this alloy, especially on catalytic activity and the structure as it related to catalytic activity, but no references were noted on melting and casting. This work was undertaken to develop the melting process, to determine the feasibility of casting, and to measure mechanical and physical properties relevant to melting and casting.
Experimental Procedure
Raney nickel alloy for this investigation was prepared by melting electrolytic nickel and 99.99 pct pure aluminum. A charge consisting of the desired amount of aluminum was placed in a graphite crucible over a portion of the required nickel on the bottom. This charge was heated by induction until the aluminum became molten and dissolved the nickel. The graphite crucible served two functions; the crucible acted as a nonreactive container for the melt and as a susceptor to help in heating the melt. The remaining required nickel was added slowly because exothermic reactions heat the melt due to formation of intermediate phases. Melting is best accomplished for the aluminum-rich composition because lower temperatures are involved and fewer intermediate phases are encountered; this is also the safest practice. Melt composition was held within 0.5 pct of the desired value using these procedures. The temperature of a melt was measured with a Pt/Pt-10pct Rh thermocouple protected by an Al2O3 tube enclosed in a graphite tube. The two protection tubes caused a lag between the melt temperature and indicated temperature, but equilibrium was obtained within a few minutes. A cryolite slag cover was used for all melts to inhibit the formation of dross and keep hydrogen pickup to a minimum.
A wide variety of mold materials was used in the investigation. The casting of rods was accomplished in Pyrex, Vycor, quartz, steel, and alumina. Sand casting of shapes was done primarily in zircon sand, but silica sand and synthetic olivine were successfully employed.
Results and Discussion
The Raney nickel alloy, as described by the phase diagram (4), is the intermetallic compound NiAl3 formed by peritectic reaction. This compound exists in the equilibrium condition but does not occur as a single phase during normal melting and casting practice. Solidification of a melt containing 42 pct Ni-58 pct Al begins at 1,080° C with the formation of the Ni2Al3 compound. At 854° C, no further Ni2Al3 is formed and the solidification of NiAl3 begins. The compound NiAl3 continues forming till 640° C is reached, when the remaining liquid portion of the melt freezes as eutectic
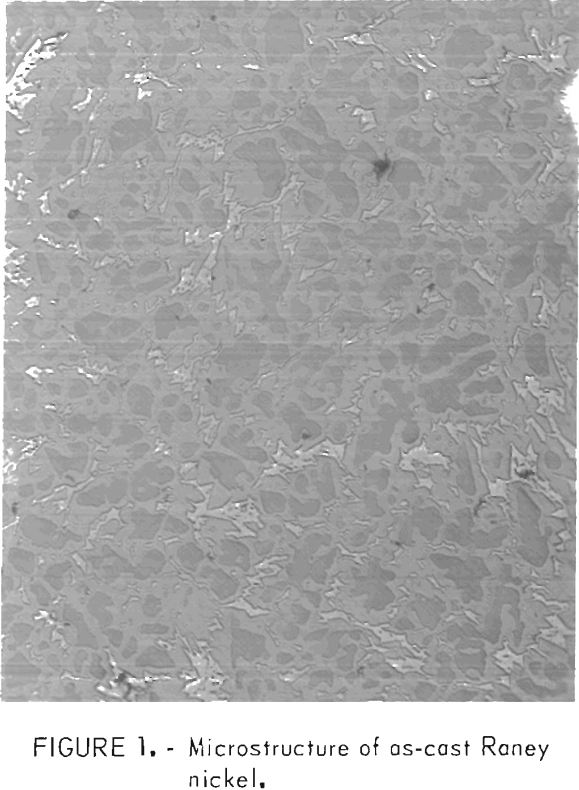
composed of Al and NiAl3. A typical as-cast microstructure is shown in figure 1. The dark inner phase is the compound Ni2Al3. The optically lighter phase surrounding the Ni2Al3 is the compound NiAl3. The third phase, the optically lightest, is eutectic. This structure illustrates the solidification pattern of first Ni2Al3, then NiAl3, and then the eutectic.
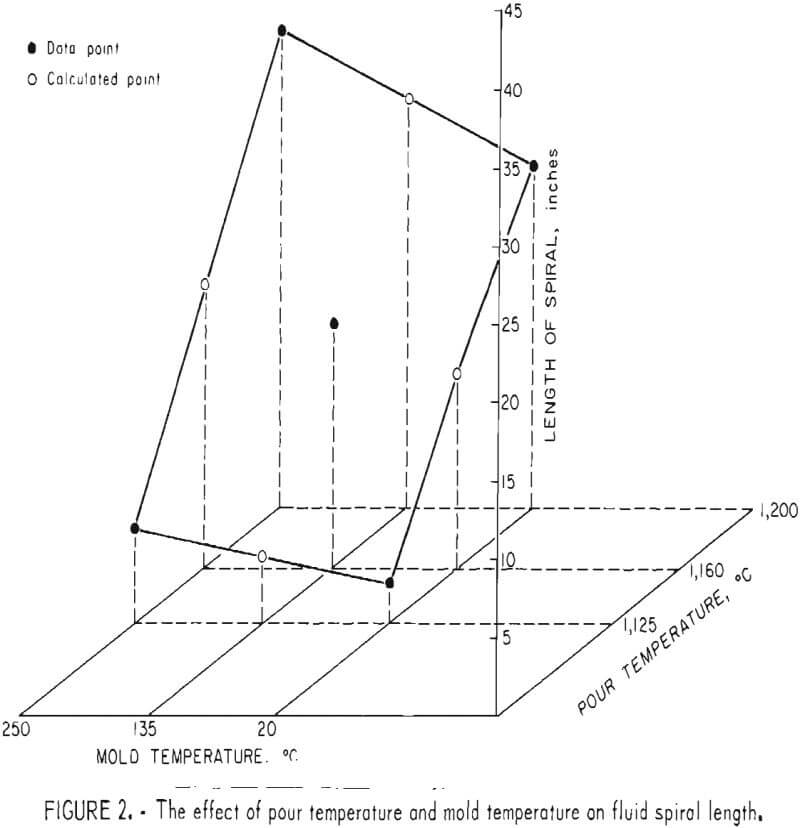
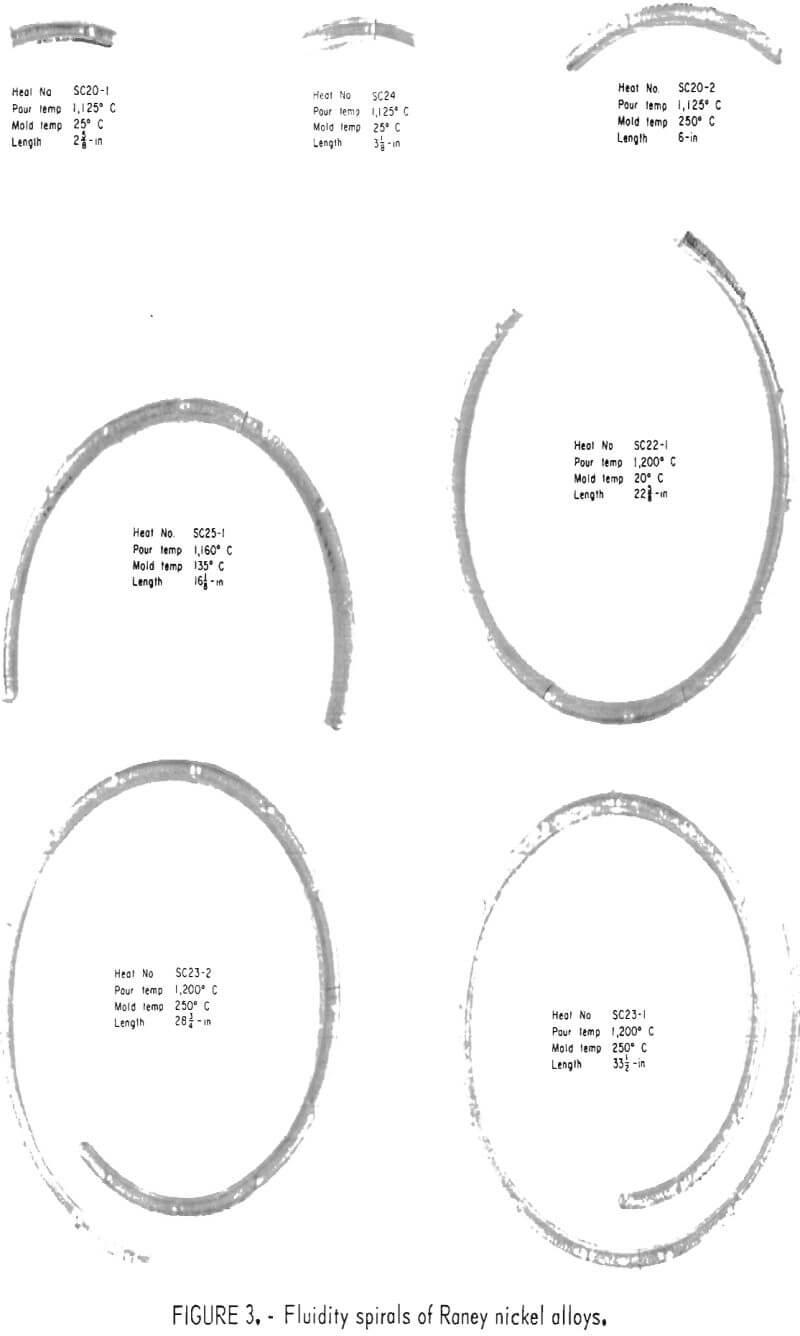
Evaluation of the fluidity of the alloy as affected by pour temperature and mold temperature was conducted using the standard fluidity spiral described by Taylor, Rominski, and Briggs. The initial pour temperature selected was 1,050° C, at which about 85 pct liquid and 15 pct solid could be expected. A pour at this temperature was impossible because of the viscosity of the liquid portion. The authors found that viscosity of the melt at 1,125° C had decreased to the point where a practical pour could be made. Results of the study are shown graphically in figure 2, and the actual spirals are illustrated in figure 3. Spirals were poured at melt temperatures of 1,125°, 1,160°, and 1,200° C and mold temperatures of 25°, 135°, and 250° C. Duplicate tests were conducted at 1,200° C melt 250° C mold temperatures and 1,125° C melt 20° C mold temperatures with excellent reproducibility. To cast a massive section one should use a pour temperature of 1,125° C with a mold at ambient or slightly elevated temperature. A melt at this temperature would have adequate fluidity to fill the mold volume and keep dross formation to a minimum. However, to fill a small cross-section mold, a pour temperature of 1,200° C would be required. The mold temperature would also have to be elevated if an intricate shape was being cast.
A pour temperature of 1,200° C and mold temperature of 250° C were chosen to produce cast 3/16-in-diam rod. The casting technique consisted of sealing a tube of Pyrex, Vycor, or quartz on one end and attaching the other end to a vacuum system. The tube was evacuated to 30 in Hg vacuum, and then thrust into the melt with sufficient force to break the sealed end on the bottom of the crucible. Metal flowed up the tube approximately 3 ft. Solidification occurred very rapidly in the tube after removal from the melt. The low softening temperature of Pyrex produced crooked rods. Vycor and quartz tubes were satisfactory and produced straight rods. A reaction between the alloy and the tube occurred if the tubes were not first coated on the inside with a graphite
or alumina wash. Mold removal occurs during the cooling by an apparent volume expansion of the alloy that fractures the tube. Casting into steel tubes was unsatisfactory because of cold shuts caused by the rapid removal of melt temperature due to higher thermal conductivity.
Raney nickel rod cast by this suction technique makes excellent feedstock for flame spraying the catalyst in the method described by Haynes, Elliott, Youngblood, and Forney. Catalytic properties of suction-cast rod used as-is instead of a coating are excellent. Test samples had a carbon monoxide conversion of 98.3 to 100 pct. Selectivity of the catalyst was favorable with conversion ranging from 95 to 98 pct CH4, 2 to 3.5 pct CO2, and 0.2 to 0.5 pct C2H6. Life testing in a pulse reactor showed no loss in activity between the 5 and 21 pulse.
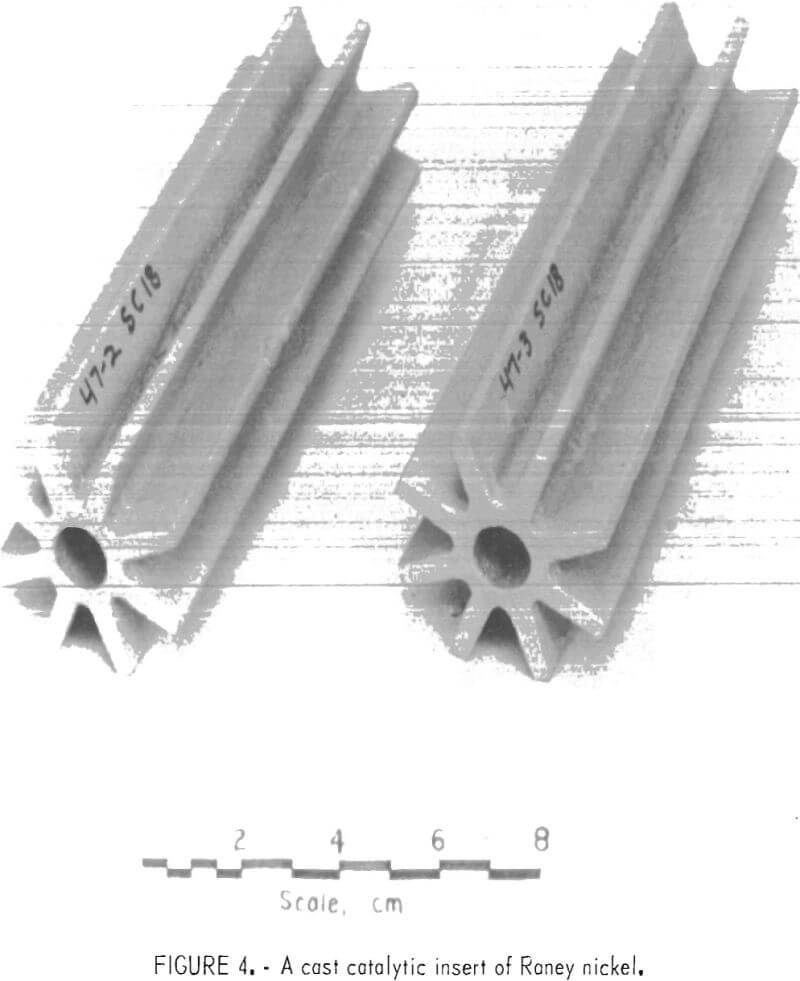
Sand castings of the alloy were poured at 1,125° to 1,150° C with the molds at room temperature. Figure 4 illustrates the type of test castings. The sand normally used was zircon because of availability, but castings in silica and synthetic olivine were satisfactory.
Mold removal from castings must be done with care. Tensile strength of the as-cast alloy is 2,000 lb/sq in with no measurable elongation or reduction
in area. Impact strength is less than 1 ft-lb. The brittle nature of the alloys prevents shakeout of a casting from the molds in a conventional manner. The best removal of a casting from a mold was found to be as soon after pouring as possible, when the mold temperature was approximately 200° C.
During solidification the molds were cracked by a volume expansion of the alloy. Because of this cracking, extremely weak molds were required to prevent hot tearing. The mold mix used for the casting illustrated in figure 4 was 2 kg zircon, 20 g flour, 20 g bentonite, and 20 ml waterglass.
The welding of Raney nickel is desirable in producing longer lengths of cast rods, repairing casting defects, and assembling castings into more complex shapes. The alloy was found to be amenable to welding using heliarc techniques. Butt welding of rods was accomplished by using high frequency for starting the arc, then welding at 132 amp. Rods up to 12 ft long were made by this technique. To weld flat sheets 1/8 in thick together, amperage was increased by 10 pct. Gas cover for both types of welding consisted of a mixture of 60 vol-pct argon–40 vol-pct helium at a flow rate of 6 cu ft/hr. No significant difference was noted in the gas content of the parent metal or the weld metal.
Microstructure of the weld was the same as observed for as-cast metal. The activation and catalytic behavior should be excellent but was not determined.
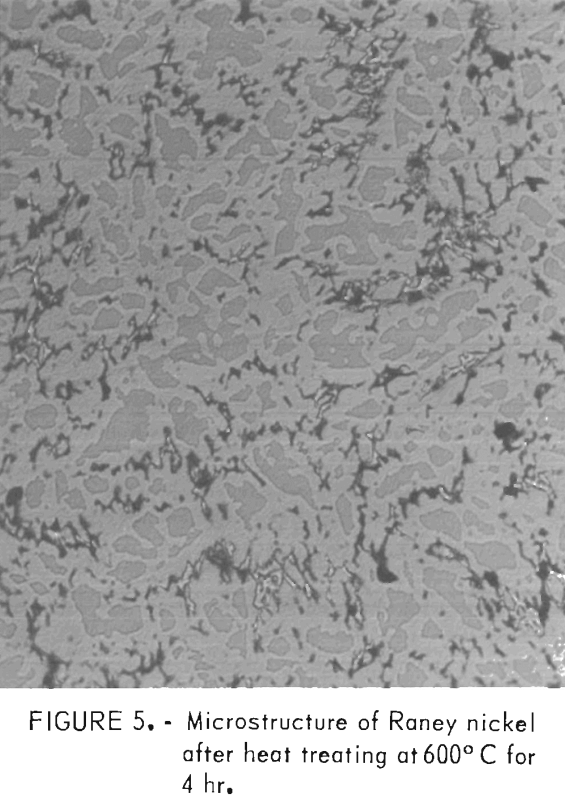
Heat treating of the as-cast alloy to promote the formation of the NiAl3 phase, which is reported to be desirable from a catalytic standpoint, was done at 600°, 700°, and 800° C. The formation of this phase and either Ni2Al3 or Al was accomplished after a 4-hr heat treatment as determined by X-ray diffraction. The Ni2Al3 or Al phase present beside the NiAl3 is determined by the overall composition of the alloy. The NiAl3 is a line compound with a precise composition, while a melt could be controlled within 0.5 pct of the aluminum content. If the melt is slightly rich in nickel, the Ni2Al3 compound is left; if lean in nickel, the Al phase remains.
All microstructures observed after heat treatment contained voids. These voids were also observed in the heat-affected zone adjacent to welds. A typical void microstructure is shown in figure 5.
Formation of the voids is attributed to Kirkendall diffusion caused by the following solid-state reaction that was observed with a hot-stage microscope:
3Al + Ni2Al3 → 2 NiAl3.
Voids also have been reported in the Ni-Al system by Itin, Naybozodenko, and Ushakov.
Thermal expansion was measured on the alloy in the as-cast condition between ambient and 500° C, and after heat treating at 550° C. The as-cast condition has an expansion coefficient of 18.4 x 10 -6/° C. In determining this coefficient, the authors found a permanent linear expansion of 2.8 pct after 6 hr at 550° C. The microstructure of the heat-treated sample is similar to the one shown in figure 5. Thermal expansion of the expanded alloy was determined to be 14.7 x 10 -6/° C.
This permanent volume expansion also occurs when the casting cools slowly in a mold. When this occurs, hot tearing of the casting results, unless a weak mold mix is used or selected breakage points are incorporated in the mold
Although void formation is undesirable in most casting, it is not felt to be detrimental for a catalyst. Porosity will enhance the rate of activation because of increased surface area, and the activated catalyst has a porous structure with a surface area in the order of 50 sq m/g.
Conclusions
The Raney nickel alloy has been studied to determine the feasibility of melting, casting, and welding and found to be amenable. Melts should have a cover slag of cryolite to prevent excess drossing. Pour temperatures of 1,125° to 1,200° C are required, depending on the cross section of the casting, with more massive casting cross sections being poured at 1,125° C.
Heated molds are desirable; a temperature range of 20° to 250° C is adequate. The molds used must be extremely weak to prevent hot tearing. This hot tearing is caused by two factors: (1) A weak brittle alloy and (2) a volume expansion caused by a solid-state reaction. This volume expansion is the result of Kirkendall diffusion and can amount to an increase of 2 to 8 pct depending on the cooling rate of the casting. Thermal expansion of the alloy is substantially different in the as-cast and heat-treated conditions.
Butt and lap welding of the alloy is readily accomplished using heliarc techniques. The heat-affected zone adjacent to the weld has a microstructure containing voids. This same microstructure with voids occurred by heat treating in the temperature range of 600° to 800° C. Phases present in the heat-treated structure are NiAl3 plus traces of NisAl3 or Al depending on the overall melt composition.
Activation and activity of the cast catalyst are excellent, as is the selectivity.
The use of cast Raney nickel alloy as a methanation catalyst in the production of a clean, high-Btu gas is possible since the alloy was found to be suitable for melting and casting.

