Table of Contents
A study has been made of the reaction mechanisms occurring during the salt-roast process to form water-soluble sodium vanadate compounds from the vanadium oxide compounds present in vanadium-bearing ores and concentrates. Two reactions have been identified, either of which lead to water-soluble compounds oxidation of sodium chloride by water vapor and oxidation of sodium chloride by oxygen. The former reaction has been found to be predominant in the salt-roast process.
Description of Process
The process, as practiced today, is briefly as follows: The ore is crushed to about minus fourteen mesh and mixed with crude sodium chloride according to the formula: six percent sodium chloride, plus one percent sodium chloride for each one percent, of equivalent vanadium pent-oxide contained in the ore. The ore-salt mixture is roasted in multiple-hearth furnaces or a rotary kiln at about 850 degrees centigrade for a period of from one to two hours at temperature. The hot calcines issuing from, the furnace are quenched into water,whereupon the water-soluble portion of the calcines is leached from the solids. Under the most favorable conditions 70 to 80 percent of the vanadium can be extracted from the ore during the water leach. The residue from the water leach is then treated with dilute sulfuric acid whereupon approximately 90 percent of the uranium and 50 percent of the remaining vanadium are taken into solution. After filtration of the barren solids from the acid leach the acid liquor is treated for the separation and recovery of uranium and vanadium.
Technical Discussion
The fundamental requirement of the salt-roast process is the formation of a water-soluble sodium vanadate through the inter-reaction of sodium chloride, oxygen and/or water vapor in the air, and the vanadium oxide species contained in the ore or concentrate. The vanadium mineralization in an ore can consist of a number of complex vanadium-bearing minerals containing vanadium in one or more oxidation states and compounded with other alkaline or less acidic metallic oxides. For the purpose of a detailed mechanism study the simplest vanadium oxide species, vanadium V oxide, has been chosen as the vanadium reactant. Thus, the over-all reactions for the solubilization of vanadium can be written as follows:
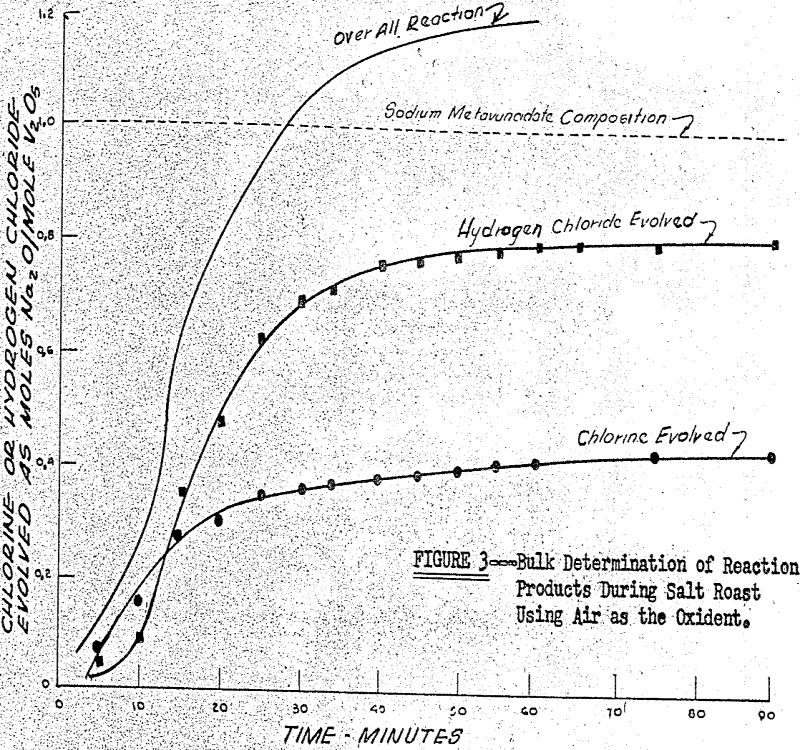
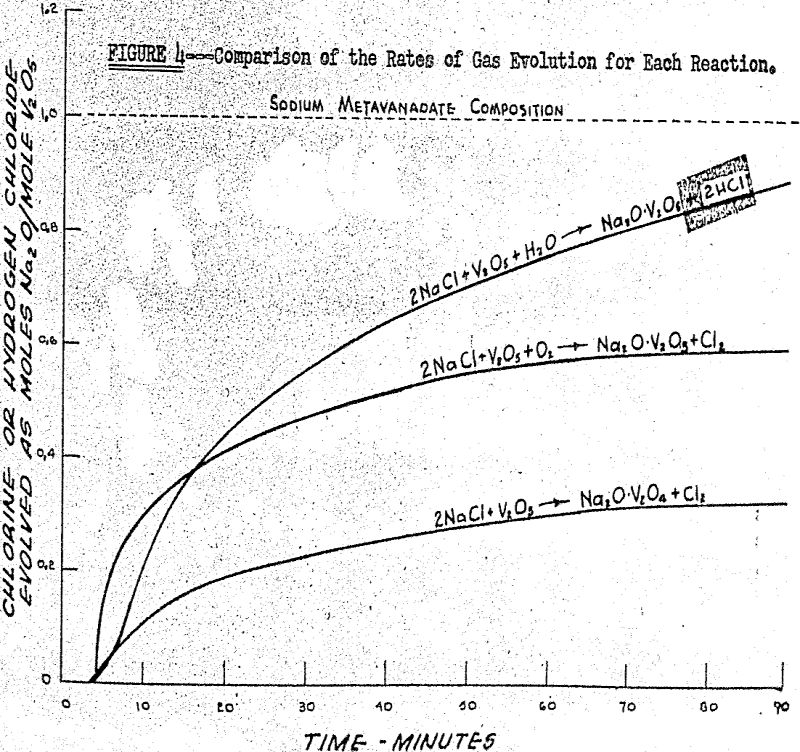
2NaCl + V2O5 + ½ O2 → Na2O·V2O5 + Cl2
2NaCl + V2O5 + H2O → Na2O.V2O5 + 2HCl
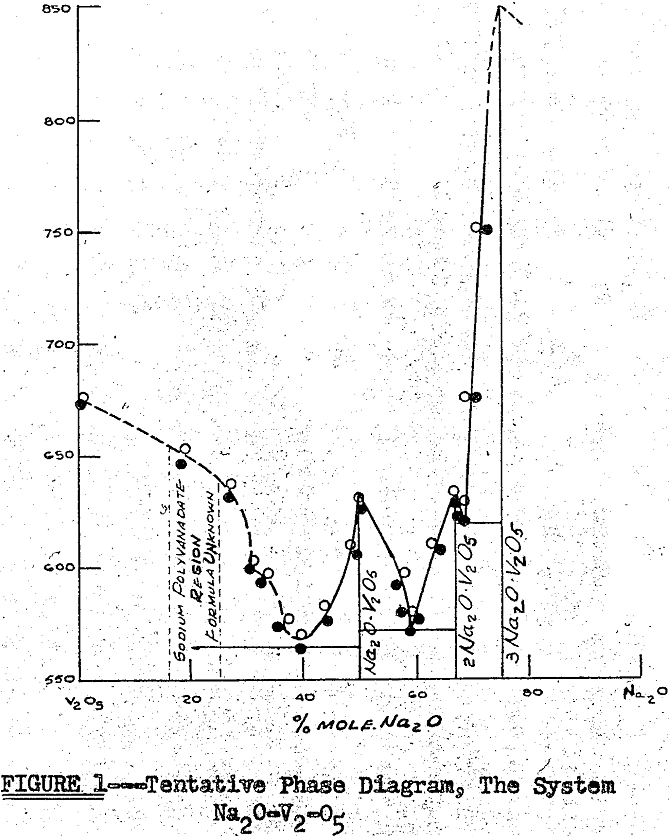
Thus, referring to the tentative phase diagram in Figure 1, the progress of a salt roast can be represented by a point moving from left to right at the roasting temperature. The point itself represents the average composition of the mixture. The actual composition of the mixture with respect to the phases present can be determined by means of the lever principle.
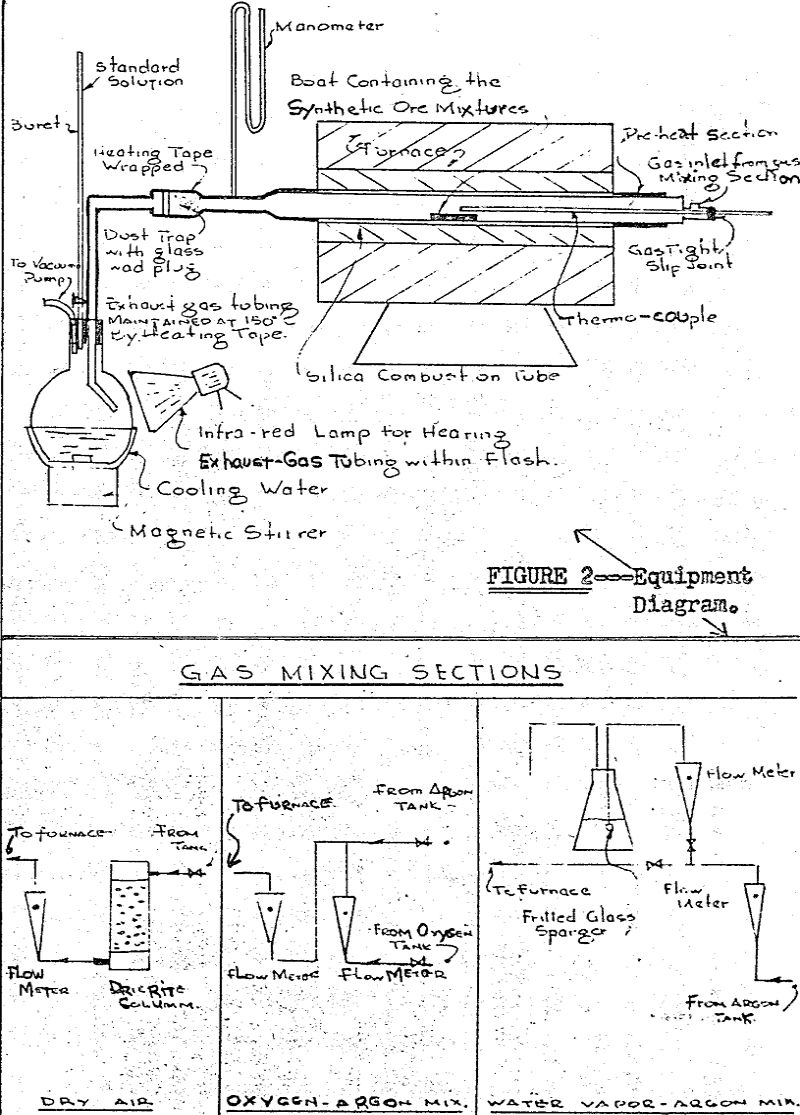
The sample under test consisted of approximately ten grams of a synthetic ore mixture containing a mechanical mixture of 3.3 per cent V2O5 in a matrix of granular silica to which NaCl was added in the amount specified by the individual experiment.
The variation of the reaction with temperature indicates several points of interest. First, the reaction does not progress at a measurable rate below the melting point of vanadium pentoxide (670°C) the lowest melting component of the system. This indicates that a solid-solid-gas reaction is quite unlikely in the salt roast process. Second, the reaction rate for the formation of sodium metavanadate is nearly doubled at the melting point of sodium chloride (800°C) due to the entrane of a second liquid phase into the system.
The fact that the melting of sodium chloride has such a great effect on the rate of the reaction forming sodium metavanadate indicates that sodium chloride must enter into the rate-controlling step of the reaction. Likewise, since the melting of sodium chloride has no effect on the rate of the reaction forming sodium pyrovanadate, sodium chloride does not enter into the rate-controlling step of this reaction.
The experimental evidence which has been presented indicates that the main rate-controlling step for the formation of sodium metavanadate by the use of oxygen is the diffusion of oxygen to the reaction site or the diffusion of chlorine away from the reaction site. Experimentally it is impossible to differentiate between the two diffusion steps of the reaction sequence. The diffusion processes followed are the stepwise diffusion through homogeneous reaction product followed by diffusion through reaction product containing voids. These voids appear when the reaction product film reaches a critical thickness.