The widespread occurrence of manganese in low grade oxide and carbonate ores not amenable to mechanical concentration has led to extensive investigations of hydrometallurgical methods for producing a pure manganese compound suitable for further treatment. Manganese carbonate is the preferable compound. This product when fully crystalline and of not too fine crystal size is easily filtrable and stable in air. It can be converted to other compounds such as the oxides by heating in air or can be reacted with acids for producing salts.
There are many data in the literature on the reactions between ammonia and aqueous solutions of manganese salts. Some of these data are apparently contradictory because of the failure to state the proportions of all constituents and the solid phase, if any, precipitated.
It may be considered as well established, however, that when ammonia is added to manganous salt solutions, cationic complexes of manganese and ammonia are formed of the type Mn(NH3)n++. According to Firth, who has made the most complete investigation of the system, the equilibrium conditions for the reaction between MnO and ammonium salt solutions can be approximated on the assumption that four cat- ionic complexes are formed, Mn(NH3)++, Mn(NH3)2++, Mn(NH3)3++ and Mn(NH3)4++
No direct data are given by Firth concerning the complexity of his manganese-ammonia solutions. The precipitation of the solutions by a freshly prepared saturated solution of ammonium carbonate, however, shows that the manganese ion concentration is greater than that corresponding to the solubility of manganous carbonate.
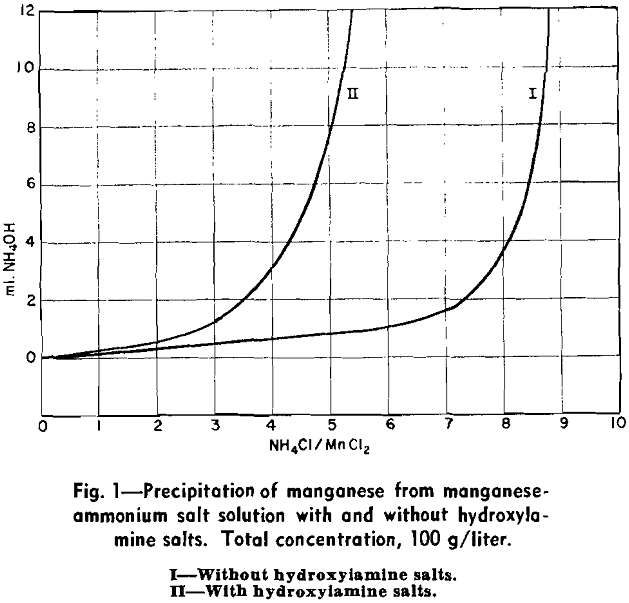
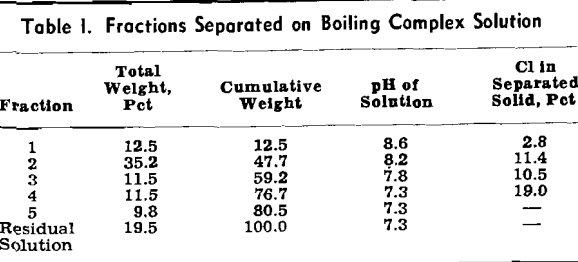
In the complete absence of oxidation, ammonium salts in moderate concentrations increase the amount of ammonia that must be added to precipitate manganous hydroxide. When air is present, the apparent amount of ammonia required for precipitation of a solid phase in the presence of a given concentration of ammonium salts is decreased. This may be caused by the impossibility of preventing local ammonia concentrations and the immediate oxidation of manganous hydroxide precipitated by these local concentrations to bring about an effective irreversibility of such precipitation.
As a typical example of a system exhibiting the presence of the new manganese-ammonia complex in solution, the manganous hydroxide, ammonium hydroxide, hydrochloric acid system will be considered first.
The phase fields of this system have been approximately determined and are plotted as a ternary system with a total concentration of the three components of 500 g per liter. This diagram represents practical equilibrium, that is, the state reached in a period of a few hours.
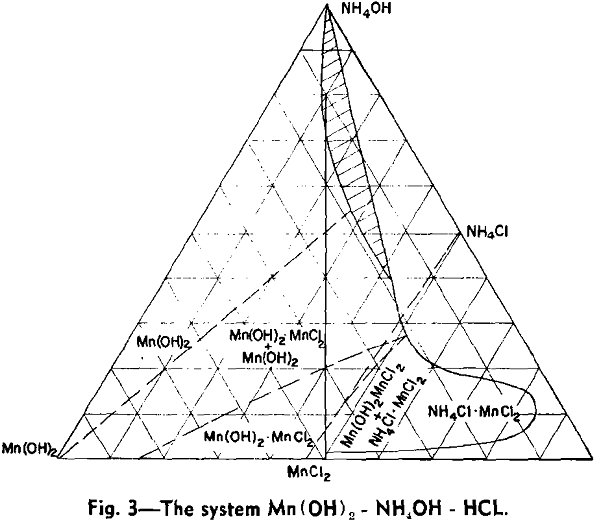
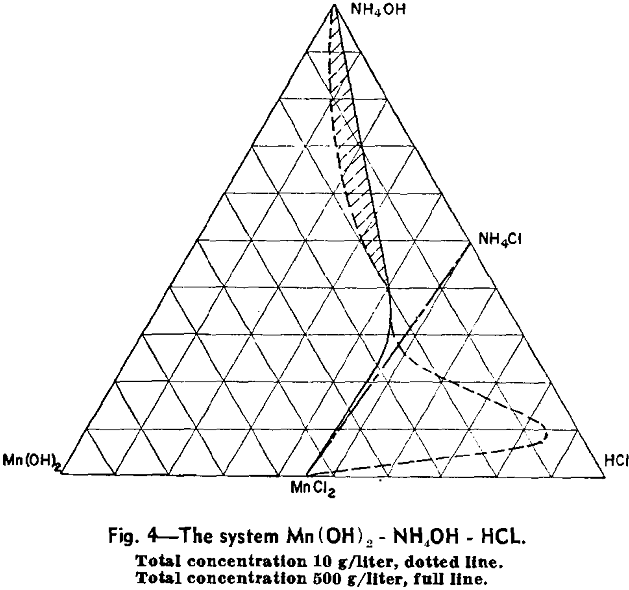
Preparation of Mn(OH)2: It is obvious that if ammonia is removed from the anionic complex by boiling, the products precipitated will be indicated by the path of a line drawn from the ammonia corner of the ternary diagram through the composition of the complex solution.
It will be seen that only the first 12.5 pct of the manganese is precipitated as Mn(OH)2 relatively free from chlorine. As a practical means of making Mn(OH)2, the manganese is substantially all precipitated and then leached with 20 pct NH4OH in sufficient amount to bring the total composition into the field in which pure Mn(OH)2 is the solid phase. No manganese but all chlorine is removed from the solid phase in this way.
Although the sulphate and nitrate systems can be used. An examination of the diagram of the acetate system shows that hydroxide can be separated only in a very small area and removing additional ammonia brings about re-solution. Such re-solution will, of course, take place in removing the ammonia from any system where the resulting composition is in the liquid phase field.
For example, in the sulphate system, the Bradley process depends on the reaction:
Mn(OH)2 + (NH4)2SO4 → Mn(NH3)n SO4 + NH3↑
Therefore, it appears highly probable that a manganese-ammonia complex is formed with the carbamate ion and that carbonate ion is present in relatively small amounts. The equilibrium is shifted toward carbonate by heating, or dilution, so that in this way, the carbonate ion reaches a concentration where manganese carbonate is precipitated from the complex, resulting in a further shift of equilibrium and eventual complete precipitation of manganese carbonate without substantial removal of ammonia.
The amount of manganese which can be built up in the pregnant solution is increased by ammonia and CO2 concentration of the solution, minimum concentrations of 14 mols per liter NH3 and 2.5 mols per liter CO2 are necessary, but little advantage is obtained by increasing ammonia above about 15 mols per liter and CO2 above 3.00. There is no disadvantage, however, in carrying NH3 to saturation (17 to 18 mols per liter) and CO2 to 4.0 mols per liter.
The Precipitation Step: The manganese from the solution containing the complex manganese carbamate can be quantitatively precipitated by heating under pressure. This regenerates the leaching solution. As a practical matter, however, the precipitation is carried out by simultaneous heating and ammonia evolution. If the ammonia is removed by heating at atmospheric pressure, the manganese will be reduced to less than 2 g per liter by lowering the ammonia concentration to about 11 mols per liter.

