Table of Contents
- Previous Studies of Magnetic Separation of Pyrite from Coals
- Present Studies of Magnetic Separation of Pyrite from Coals
- Introduction
- Size Distribution of Pyrite Particles in Coals
- Magnetic Susceptibilities of United States Coals
- Magnetic Susceptibilities of Some Compounds of Iron
- Enhancement of Magnetism of Pyrite
- Effect of Storage on Sulfur Forms
- Effect of Pulverization of Coal and Pyrite
- Magnetic Susceptibility and Separation of Untreated Coals
- Magnetic Susceptibility and Separation After Treatment in a Fluidized Bed
- Summary of Results and Conclusions
- Selective Heating of Pyrite in Coal
- Introduction
Exploratory measurements of the dielectric constant of coal and pyrite indicate that pyrite in coals can be heated selectively. If about 1 percent of the radiation is absorbed in the process, it would be very attractive from a commercial standpoint. The following recommendations are offered.
- More precise and systematic measurements of the dielectric properties of coals in the Mhz and Ghz region are needed. If there exists an absorption peak for pyrite, it may dictate that heating be done at the frequency of the peak.
- Systematic continuous heating of coal and pyrite should be investigated at frequencies 2.45 Ghz and X-band inasmuch as high intensity generators at these frequencies are available. Such studies should be made with the objective of determining the desired intensity levels, sample thickness, belt material, belt speed, induced susceptibilities, etc.
In this type of heating, pyrite need not be crushed to be reactive; indeed, the opposite is true, that is, the coarser the pyrite, the more readily it will be heated. Crushing process necessary to liberate pyrite can be done after dielectric heating. These factors should be investigated systematically.
In recent years air and water pollution resulting from mining and burning of coal has come under public scrutiny. Earlier concern was over the smoke produced from coal-burning installations. Efforts were directed toward more complete combustion in powerplants, development of processes for smokeless fuel for domestic use, and reduction of dust effluent from chimneys. More recently, sulfur in coals and rocks overlying coalbeds has received wide attention as a major cause of air and water pollution. Sulfur content of coals used by public utilities for steam and electricity generation ranges from about 1 to 5 percent. In 1963, for example, 209 million tons of coal containing on the average 2.5 percent sulfur was burned in the United States; the sulfur discharged into the atmosphere, mainly as sulfur dioxide, amounts to about 5 million tons annually. Considering the projected increase in demands for electric power, the statistical information cited to demonstrate the seriousness of the problem is impressive. As a result we see lately the enactment of State and Federal legislation leading to regulations placing upper limits on the sulfur content of coals to be burned in powerplants or on sulfur dioxide content of the discharged flue gas.
The problem of pollution by coal directly involves its utilization as a source of power and hence its value as a natural resource. Prevention or alleviation of pollution connected with coal utilization has been the subject of numerous studies by the U.S. Bureau of Mines. As pollution came into the public limelight, studies of the subject assumed a very prominent part of the Bureau’s research and development program on coal. Any additional processing of coal before it is burned or processing of the flue gas adds to the cost of the product, for example, electricity; the amount added may be lowered by byproduct recovery. For coal to be competitive in the future power picture, the cost of removal of sulfur must be kept reasonably low. For this reason several processes of sulfur removal are being considered. Eventually a cost analysis will determine the process or combination of processes to be used. Locality, of course, will also be an important factor in the choice.
A solution to the problem is the removal of sulfur dioxide from flue gas. This approach has been studied at the Bureau of Mines for a number of years, culminating in the development of a dry scrubbing process that utilizes regeneratable alkalized alumina catalysts. The process has received worldwide attention. A pilot plant has been constructed for cost evaluation. Other catalysts are being investigated presently.
An alternate or corollary solution to the problem is the removal of sulfur before coal is burned. In coals containing more than 2 percent sulfur, about 1 percent is intimately tied with the structure of coal; hence it is referred to as organic sulfur. The remainder is in the form of sulfides of iron; it is called pyritic sulfur. Although studies have been conducted for the removal of all forms of sulfur in coals, it is recognized that the physical properties of pyrite are very different from those of coals; thus, emphasis is directed toward removal of pyrite from coal.
One of the methods considered for pyrite removal is the conventional cleaning of coarse coal by density separation. Coals contain mineral matter to a varying degree. Mineral matter dilutes coal and reduces its calorific value. Transportation costs for long distances often dictate that coal be cleaned before it is shipped; as a result cleaning of coal to reduce its mineral matter content has become an established process. The process is being reinvestigated to increase the efficiency of pyrite removal.
If pyrite is finely distributed in coal, it becomes necessary to crush the coal to liberate the pyrite. Removal of the liberated or partly liberated pyrite by the conventional techniques of wet ore processing has been the subject of many studies. One of the processes is froth flotation; it has not proved to be effective as yet. Gravity methods, on the other hand, suffer from low rates of settling of fine particles in liquids. Dry methods that have received attention are air elutriation, electrostatic separation, and magnetic separation. Investigation of these methods for pyrite removal has been on a limited scale. The study reported below concerns the magnetic separation of pyrite from coal.
Previous Studies of Magnetic Separation of Pyrite from Coals
Published Work
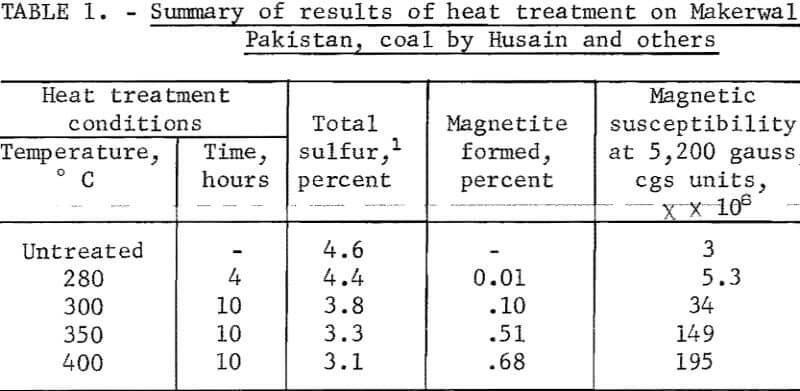
Siddiqui in 1957 steam-treated Makerwal, Pakistan coal for 4 to 10 hours at temperatures 200° to 300° C and magnetically separated the treated coal. He reported beneficiation by separation after treatment. Husain and others extended Siddiqui’s studies; their results are summarized in table 1. The coal used contained a large amount of organic sulfur, that is, 3.4 percent organic sulfur compared with 1.2 percent pyritic sulfur. A small horseshoe magnet was used to remove 8.6 percent of the sample treated at 400° C, for which chemical analysis gave 7.2 percent total sulfur. Evidently the beneficiated fraction contained 2.7 percent total sulfur, well below the organic sulfur of the untreated sample. X-ray diffraction patterns of the sulfur-rich component were reported to reveal the presence of FeS, Fe3O4, and kaolinite. The report of magnetite formation is very interesting. However, the excessive reaction time required renders the process uneconomical.
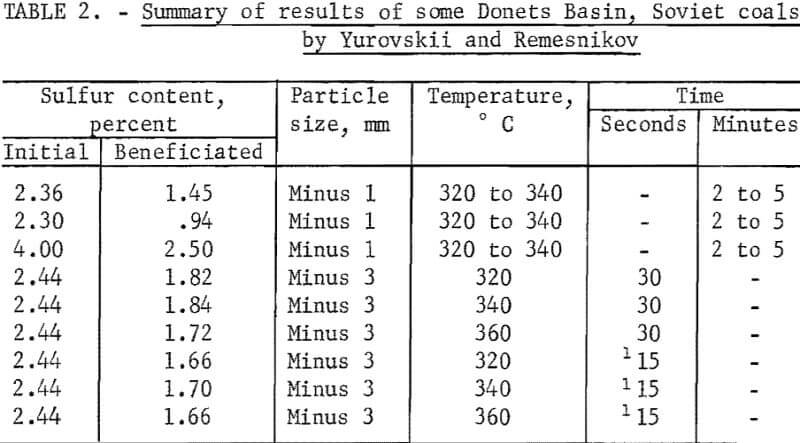
Yurovskii and Remesnikov used steam-air treatment of some Soviet coals at temperatures ranging from 120° to 360° C for relatively short times, from 15 seconds to 5 minutes. The schematic diagram indicated that magnetic separation was accomplished by means of the pickup mode of separation using an induced roll magnetic separator. The results of their studies are shown in table 2. It is seen that appreciable reductions in sulfur content were reported upon treatment and separation. Steam was probably not used in 15- and 30-second tests because they were designated to simulate quick heating in a turnover chamber that was part of a new coking process. No X-rays or magnetic susceptibility studies were made by the authors. Possible surface minerals on the pyrite were listed as magnetite, gamma-hematite, pyrrhotite, and ferrous and ferric sulfate. Chemical analysis indicated that 2.5 to 4.5 times more sulfate sulfur was present in the magnetic fraction than the beneficiated fraction. The low time requirement of their study is very advantageous from the standpoint of a commercial process.
Adamov used powdered magnetite, <56 µ as an activating additive for cleaning pulverized coal, minus 3 mm in size, using a magnetic separator. The ash content was decreased by about 3 and 10 percent when the beneficiated fractions were 90 and 55 to 60 percent, respectively. For 10 percent recovery of the original sample, the average decrease in the ash content was reported to be 7 percent on an absolute basis, 45 percent relative.
Kester thermally treated an Upper Freeport bed coal for 5 to 10 minutes at temperatures ranging from 175° to 280° C at various air to steam ratios. Two closely sized sieve fractions, that is, 297 to 210 µ and 149 to 105 µ, were utilized in magnetic separations rather than the total pulverized coal sample. For the 297 to 210 µ, fraction the best reduction in total sulfur was 38 percent; the results with the 149 to 105 µ, fraction were erratic. Raw, untreated coal also was magnetically separated for comparison with the results above. Five samples of the coarser size fraction resulted in 42 to 49 percent reduction in total sulfur content with accompanying reductions of 31 to 44 percent in ash content. Separation of eight samples of the finer size fraction produced 47 to 57 percent reductions in sulfur content and 26 to 44 percent reductions in ash content. The beneficiated fractions ranged from 84 to 91 percent of the initial sample. Kester concluded that superior coal quality and separation efficiency was obtained by using untreated coal samples. Kester’s work was extended to three other coals by Kester, Leonard, and Wilson. Their procedure is not adaptable for large —scale commercial operation. The separation was done using a vibrating slanted track mode of an isodynamic magnetic separator; it is an extremely slow operation, requires close sizing, and is not suitable for fine coal. We note a conclusion by them that “48- x 0-mesh size coal yielded erratic and often ineffective results”. All of their cleaning studies were confined to 48- x 200-mesh (297 to 74 µ) coal size fractions. It should be noted that coal burned by the electric utilities is pulverized so that 70 to 90 percent passes through 200 mesh. Kester’s results with steam-air treatments are interesting; we see a conflict with the results of Yurovskii and Remesnikov.
Cindea-Muntean and Gonteanu found that for Rumanian coking coals containing 2.8 to 3.2 percent sulfur, larger fractions of beneficiated coals could be obtained by magnetic separation than by centrifugal washing. However, the quality of the product was poor. They observed also that thermal oxidation treatment produced no improvement in the separation.
Unpublished Studies by the Bureau of Mines
Starting in 1961 isolated studies were performed by the Bureau of Mines on the magnetic separation of pyrites obtained from different sources using an isodynamic separator on vibrating slanted track mode. The first series of experiments involved pyrite from Pittsburgh bed coal, collected from a wet concentrating table of a coal preparation plant. The samples were air-dried and 150 to 210 µ, size fractions were treated with steam-air at 300° C for various times. An untreated sample yielded a magnetic fraction of about 13 percent. Little improvement was observed with heat-treatment periods up to 2 minutes. The 5-minute treatment resulted in a 72-percent magnetic separation; after 15 minutes the magnetic fraction was 55 percent, suggesting that there was an optimum time, about 5 minutes, for increasing the apparent magnetism of the pyrite particles. The untreated sample contained 0.4 percent sulfate sulfur and no detectable ferric iron; upon 15-minute heat treatment the sulfate sulfur increased to 1.4 percent and ferric iron (hematite), to 6.9 percent. No magnetic susceptibility measurements were made for the samples.
The studies were extended to observe the influence of particle size and origin of pyrite. Several size fractions of sedimentary pyrite obtained from four coal mines and hydrothermal pyrite ore from a metal mine in Rico, Colo., were treated with steam-air at 300° C and separated magnetically. Contrary to earlier experiments heat treatment did not prove to be beneficial in most cases. For all of the pyrites minus 44 µ fractions appeared to be easily separable; however, it could not be ascertained if the separation was the result of particle size effect rather than a susceptibility effect per se.
The use of commercial dryers for heat treatment of coals and mixing of coals with magnetite to improve the magnetic separation of pyrite were also investigated. Drying of a Pittsburgh bed coal with a flash dryer (nominal rating 540° C) and of an Upper Freeport bed coal with a rotary kiln dryer did not improve the magnetic separabilities of 74 to 595 µ, size fractions of the coals. Mixing magnetite with pyrite or with coal resulted in a reverse selectivity, that is, the nonmagnetic fraction of the coal had more sulfur. Steam-air treatment of the mixtures showed no promise at all.
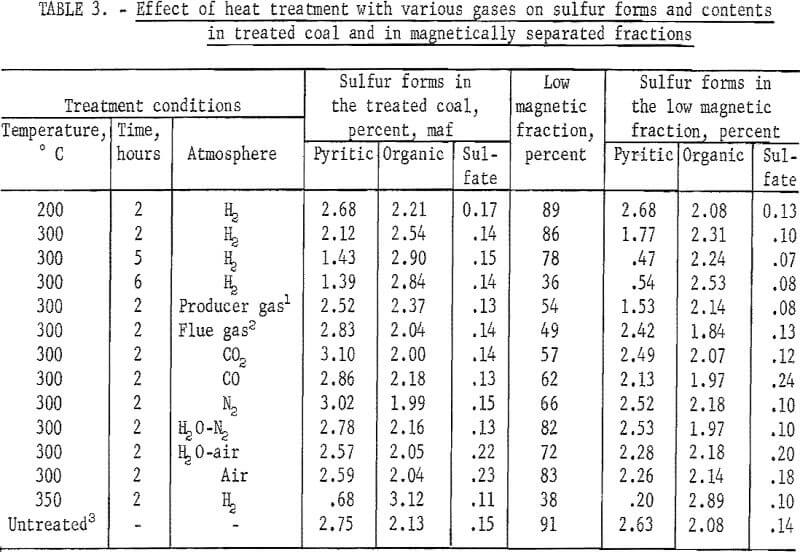
The next series of experiments involved the investigation of the effect of heat treatment, atmosphere, temperature, and residence time on the magnetic separation of a minus 48-mesh coal. Separations were made using an isodynamic magnet in a free fall mode. In table 3 are listed yields upon magnetic separation and sulfur-form analyses of the untreated and treated coal and of the low magnetic fraction. Heat-treatment temperature ranged from 200° to 350° C and residence time from 2 to 6 hours. Investigation of the influence of heat-treatment atmosphere was done at 300° C, using 2 hours residence time. The only significant reduction in the sulfur content with reasonable yield of low magnetic fraction (about 78 percent) occurred with hydrogen at 300° C using a residence time of 5 hours. In this experiment, however, over 10 percent of the sulfur in the coal was thrown into the hydrogen atmosphere. In general, residence times used in the study are too excessive for any practical process that may be contemplated.
Present Studies of Magnetic Separation of Pyrite from Coals
Introduction
The foregoing survey shows that only isolated efforts have been made to date to separate pyrite from coal by magnetic means. The results are not particularly encouraging; a few favorable results were followed by adverse observations. However, we should note that separation of pyrite from coals involves a variety of problems. First of all, pyrite particles must be adequately liberated from coal if they are to be separated by physical means. Yet no information was given about the state of liberation of pyrite particles in the various studies reported. Secondly, the basis of magnetic separation is that pyrite is more magnetic than coal and/or can be partially converted into more magnetic compounds of iron upon treatment.
Thus any separation or treatment can best be followed by magnetic susceptibility measurements which give a measure of force of separation. No genuine effort has been made in this direction. Finally, a treatment is made on the premise that a favorable reaction will occur; in the case of pyrite, partial conversion into ferromagnetic compounds of iron is expected. We really have little or no idea about the rates of formation of the various ferromagnetic compounds of iron from pyrite. In view of these complex problems, we may appreciate the preliminary nature of the studies conducted to date and the need for more elaborate studies. In the studies reported below the broad aspects of the problem received particular attention with the objective of narrowing the scope of future studies. Unfortunately, however, the experiments could not be carried out in the order in which they are presented below. The presentation is in retrospect. Separation and heat-treatment experiments could be carried out more rapidly, whereas the construction and assembly of a magnetic balance and the development of a method of analysis of pyrite distribution in coals required a considerable period of time.
Size Distribution of Pyrite Particles in Coals
As mentioned above, adequate liberation of pyrite particles embedded in coal is necessary if they are to be separated by physical means. This can be accomplished by crushing coal to a size approaching that of the pyrite particles. Usually coal is crushed so that about 80 percent passes a 200-mesh screen before it is burned in powerplants. If finer crushing is necessary, the added cost of fine crushing and problems of handling finer coal must be considered. In case the pyrite in coal is distributed so finely that crushing costs would be prohibitive, there is no sense in attempting to remove the pyrite by physical means. If, on the other hand, the pyrite particles are relatively coarse, a separation procedure may be more effective and economical if the coal is crushed only moderately before separation. The importance of a knowledge of particle size distribution of pyrite in coals is readily recognized.
Determination of size distribution of pyrite in coals has not proved to be simple. Sizes of pyrite particles embedded in coals range from submicron to several millimeters. We are, of course, concerned with particles that exist in significant amounts by weight. Earlier studies of some selected coals led to the development of techniques that involved visual microscopic examination of particles embedded in polished briquettes. A large number of particles, 1,000 or more, had to be examined to yield statistically significant results. The distinction between pyrite and coal is marked because of the much higher reflectance of the former. Workers at the Bureau of Mines pioneered in demonstrating that reflectance distribution can be obtained automatically by using a multiplier phototube attached to the ocular of a microscope, while scanning the sample at a constant linear velocity. With the advent of high-speed computers and techniques of storing a large amount of data on magnetic tapes, automatic analyses became feasible. A procedure has now been developed and tested at the Bureau of Mines for determining the size distribution of pyrite in coals and pyrite association, that is, if pyrite particles are free or partly free, and how they are concentrated in coal particles. To give an idea about what is involved in an analysis, suffice it to say that several millions of reflectance readings are necessary in the analysis of a single coal. Yet this takes only a few hours of experimental work and a few minutes of computer time.
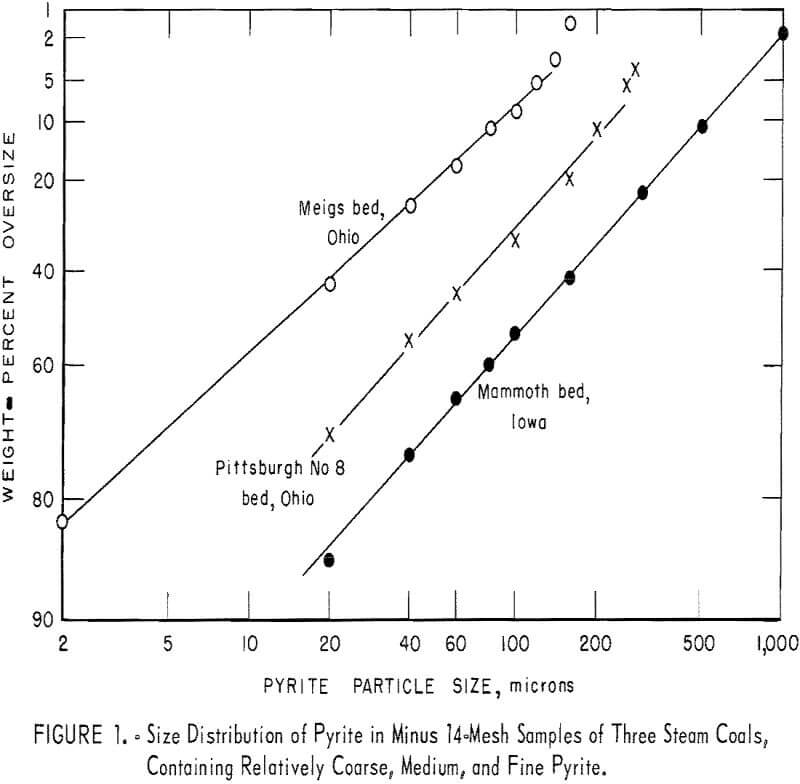
Figure 1 shows the size distribution of pyrite in minus 14-mesh samples of three steam coals, containing relatively coarse, medium, and fine pyrite. The upper limit of coal particle size, 14 mesh, is dictated by experimental factors, that is, the use of a microscope and the consequent statistical requirements. A discussion of the interpretation of the distribution observed is beyond the scope of the present study. It is sufficient to state that the efficiency of various pyrite removal processes depends greatly on the pyrite size distribution in the coal sizes treated.
Magnetic Susceptibilities of United States Coals
With the initiation of studies of magnetic separation, emphasis was directed toward the construction of a magnetic balance for low paramagnetic and diamagnetic susceptibility measurements. The instrument developed is a Faraday-type null instrument that permits a precision of about ±0.01 x 10 -6 in cgs susceptibility units. A survey of literature revealed that very few studies have been made of magnetic susceptibilities of coals and no studies have been reported on United States coals. In the course of the investigation numerous measurements were made on the samples of coals being studied. The results on seven selected United States coals ranging in rank from high-volatile C bituminous to anthracite are given below.
It is well known that many physical properties of high-rank coals, for example, refractive index, extinction coefficient, X-ray and electron scattering intensities, are-different according to whether they are measured along the bedding plane or perpendicular to it. For this reason, samples of vitrains (anthraxylons) were carefully cut, noting the direction normal to the bedding, and cubes of 5±0.5 mm were obtained. The vitrain samples were selected from relatively wide uniform bands, and they were examined microscopically to be sure that they were free from visible mineral matter. Final shaping was done by grinding with sandpaper to avoid ferromagnetic contamination. For diamagnetic measurements the balance was calibrated with water occupying the same volume as the coal specimens. Measurements were made at room temperature under a field of 6,000 gauss. The cubes were oriented so that the field was either parallel or perpendicular to the bedding plane. In the cases of anthracite and semianthracite, measurements were made along the third axis of the cube. The results are reported in table 4. No corrections were made for paramagnetic contributions. The data correspond to averages of at least two measurements on the same sample, the second measurement being made after measurements were made at least on another sample. The average deviation of all repeated measurements was ±0.02 and rms deviation was ±0.03 x 10 -6 in cgs units.
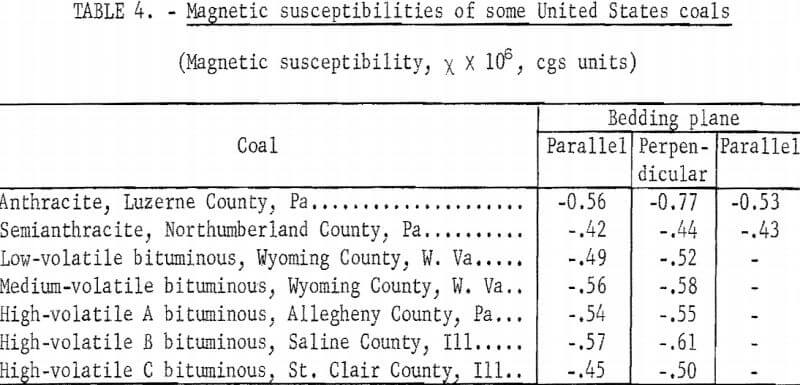
From an inspection of the table it is seen that coals are diamagnetic. Susceptibilities with the field parallel to the bedding plane indicate no special trend with rank. The perpendicular susceptibility of anthracite is significantly higher than its parallel susceptibility; that is, anthracite shows a significant magnetic anisotropy. Measurements along two axes in the bedding plane with anthracite and semianthracite indicate no anisotropy. Finally we observe that in every case the perpendicular susceptibility is slightly higher than the parallel susceptibility, the difference being very small excepting for anthracite.
The results reported here are in good agreement with those of Wooster and Wooster on British coals.
Magnetic Susceptibilities of Some Compounds of Iron
Various sulfides, oxides, and other compounds of iron are ferromagnetic or ferrimagnetic, while other compounds with the same elements are not. In table 5 are listed the magnetic susceptibilities of pyrite, iron, and other compounds of iron into which pyrite may be converted.
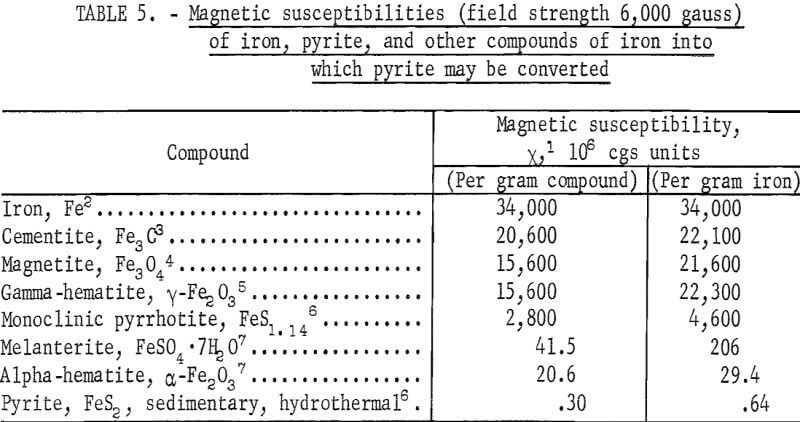
In general x varies with field strength; the values of x reported in table 5 are for H = 6,000 gauss, the field strength used in the measurements made by the Bureau of Mines. Iron and four of its compounds listed, namely, cementite, magnetite, gamma-hematite, and monoclinic pyrrhotite, are ferromagnetic or ferrimagnetic, and their susceptibilities were interpolated from values of specific magnetization, σ, reported in the literature. Melanterite and alpha-hematite are paramagnetic and pyrite is slightly paramagnetic. In measuring the susceptibility of pyrite, samples of highly purified sedimentary pyrite from coals and hydrothermal pyrite from igneous rock formations were employed. Table 5 also lists magnetic susceptibilities per gram of iron present in the mineral. The values are useful in calculating the increase in susceptibility of pyrite upon partial conversion into the other compounds listed.
Enhancement of Magnetism of Pyrite
As seen in table 4, coals are slightly diamagnetic, that is, when placed in an inhomogeneous magnetic field, they tend to move toward the weaker regions of the field. Pyrite, on the other hand, is slightly paramagnetic and moves toward the stronger regions of the field. The paramagnetism of pyrite, however, is not sufficient to effect a separation from coal. Factors such as the momentum of particles, their collisions and adhesion by electrostatic forces, surface striae, etc., oppose separation. Thus a minimum paramagnetism is required to overcome these factors and its magnitude depends upon the state of the coal, the separator used, and the efficiency of separation desired. These factors have not been studied yet. However, based on the experiments reported in the paragraphs that follow we may state that a minimum paramagnetism of 2 to 10 x 10 -6 in pyrite particles appears to be necessary to separate them from, say minus 80-mesh coal when using a large-scale induced roll magnetic separator. Imparting stronger paramagnetism to pyrite forms the basis of its magnetic separation.
Decomposition of pyrite has been studied by numerous workers. Upon heat treatment pyrite may change to a variety of sulfides of iron, FeS1 + x, ranging in composition from FeS2 to FeS, troilite, some of which are ferrimagnetic. Of these compounds FeS1.14 (Fe7S8) is known as monoclinic pyrrhotite; it is ferrimagnetic and has a large susceptibility at room temperature (Curie point at 305° C). FeS1.09 is called hexagonal pyrrhotite; it is also ferrimagnetic but is of “peak-type”; it shows a sharp susceptibility peak at 220° C. In general, decomposition reaction of pyrite becomes noticeable at temperatures above 500° C, and the reaction has a high temperature coefficient.
Reactions of pyrite in air or in the presence of other oxidizing gases yield oxides and sulfates of iron as well as sulfides. Of these compounds monoclinic pyrrhotite, γ-hematite, and magnetite have a very large susceptibility at room temperature. Whereas decomposition reactions occur at elevated temperatures, it is well known that pyrite in some coals when exposed to air oxidizes into ferrous sulfate at room temperatures. The reaction is slow but noticeable over a period of several days. In the presence of reducing gases, for example, hydrogen, decomposition of pyrite produces sulfides of iron and hydrogen sulfide. Most sulfides, sulfates, and oxides of iron have higher paramagnetic susceptibilities than pyrite at room temperature.
In table 6 are shown the apparent magnetic susceptibilities of pyrite when 1 percent of it is converted into the compounds of iron indicated. Ferromagnetic or ferrimagnetic forms result in a more than hundredfold increase in susceptibility. It is seen that a conversion of pyrite by about 0.5 percent into monoclinic pyrrhotite or 0.1 percent into the other ferromagnetic forms of iron is sufficient to produce an apparent susceptibility of 10 or more; if melanterite is formed, about 12 percent of pyrite needs to be converted. For a coal containing 6 percent pyrite, that is, 3.2 percent pyritic sulfur, a 12 percent conversion would result in an increase of sulfate sulfur by 0.19 percent based on the weight of the coal with an equal amount lost to the atmosphere as sulfur dioxide. It will be shown that increases of about this magnitude upon weathering or heat treatment of coals are not uncommon.
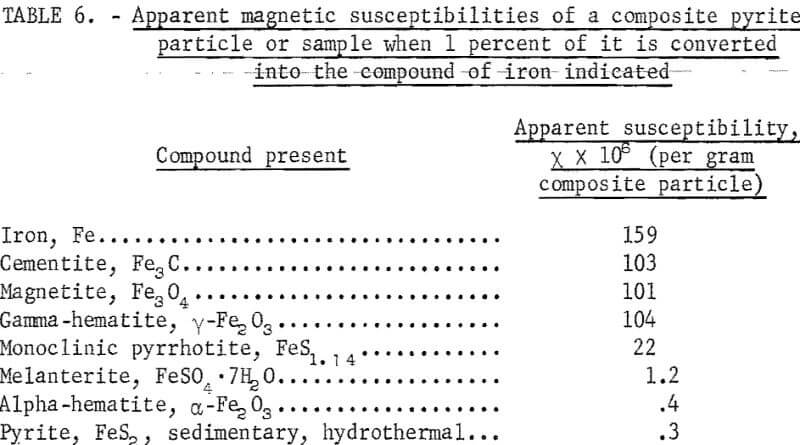
From the foregoing discussion it is seen that only a small fraction of pyrite, that is, less than 0.5 percent, needs to be converted into a form exhibiting spontaneous magnetization to separate pyrite from coal. No investigation of pyrite, however, has been made with an objective to find the conditions under which small amounts of highly magnetic compounds of iron can be formed rapidly.
Effect of Storage on Sulfur Forms
In a test period lasting 320 days, the sulfate sulfur content of a minus 48-mesh Pittsburgh bed coal sample increased on the average of 0.01 percent every 20 days (from an initial value of 0.04 to 0.20). The coal was kept in a plastic bag placed in a tight metal container. The necessary moisture and oxygen were evidently supplied by the moisture content of the coal and the air entering the bag when samples were taken for examination. Periodic checks on another sample revealed an increase of about 0.01 percent per month. A third coal left in cans for 80 days showed an increase from 0.02 to 0.08 percent. The fastest observed rate was about 0.01 percent per week. The inherent moisture content, atmospheric condition, bacteria present in the coals may have a great influence on the rate. The point to be made here is that the sulfate sulfur content of the coals increases upon weathering. Iron sulfate, being more paramagnetic than pyrite, increases the apparent susceptibility of pyrite. About a 12-percent conversion of pyrite would yield a susceptibility of about 10 x 10 -6. However, it should be noted that if a large particle, say 100 µ, oxidizes to an extent of 10 percent conversion into sulfate a smaller particle, say 20 would be converted to a much greater extent because the surface area per gram increases with decreasing diameter. Since the surface area per gram is 5 times that of the large particle, the 20 µ, particle will be 50 percent converted if reaction continues to the same depth as in the large particle and if reaction is not accelerated by higher temperature due to the increased reaction surface per gram. Thus the required overall degree of oxidation of pyrite would be much larger if there is a wide size distribution.
Effect of Pulverization of Coal and Pyrite
In. the crushing of hard particles localized pressures are estimated to be in excess of several tens of kilobars, creating localized temperatures approaching 1,000° K for crystals which have low thermal conductivity. Since pyrite decomposition has a high energy of activation, extremely rapid decomposition at localized areas may occur if such temperature conditions may be attained. It is estimated that only a small fraction, less than 1 percent, of the energy input during pulverization of coal is utilized in size reduction, that is, in producing new surfaces, the remainder being dissipated as heat. In the case of pyrite, the premise of enhancement of its magnetism by crushing is the formation of ferromagnetic components of iron on the surfaces formed during fracture. The method and mode of crushing may have some influence on the magnetism imparted.
A fresh sample of Pittsburgh bed coal was obtained directly from a coal preparation plant and was pulverized to minus 150 µ using three different commercial pulverizers: A hammer mill, a roll mill, and an impact pulverization mill. Pulverizations were conducted in air employing the usual procedures. The coal had 0.02 percent sulfate sulfur. Magnetic separation (using an induced roll separator) of the fresh coal pulverized by the three different mills yielded no significant reduction in sulfur content. After storing the samples for 80 days, the sulfate sulfur increased to 0.08 percent and magnetic separations resulted in a reduction of pyritic sulfur from 3.1 to 2.0 percent corresponding to a relative reduction of about 35 percent.
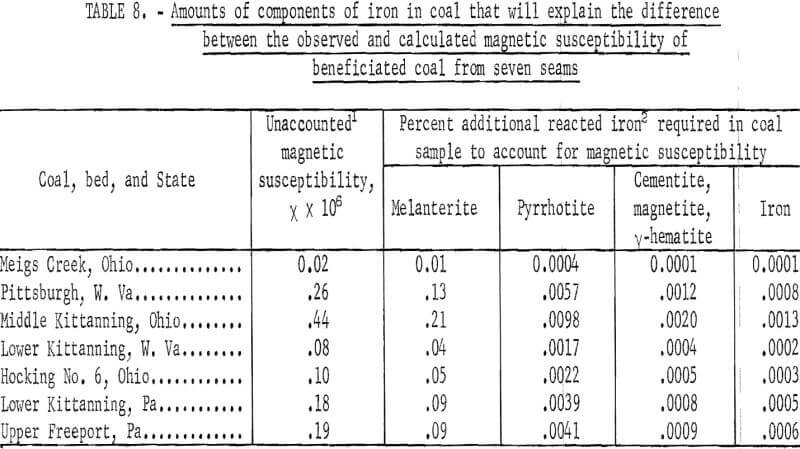
The laboratory model of induced roll magnetic separator produced beneficiated pulverized coal of high magnetic purity when the hopper provided by the manufacturer was replaced by a vibrating track so that coal was fed very evenly to the roll. Table 7 shows the magnetic susceptibility of beneficiated coal from seven seams. The largest measured negative susceptibility (that is, diamagnetic) is 0.31 x 10 -6. The positive magnetic force of attraction for pure pyrite is also low, that is, of about equal magnitude. Table 8 shows the small amount of reacted iron required to explain the difference between the observed and calculated magnetic susceptibility for coal from the seven seams. The ability of the induced roll magnetic separator to beneficiate pulverized coal to high magnetic purity, makes it a useful tool in evaluating the increase in magnetic susceptibility of pyrite embedded in coal due to pulverization by various techniques. The failure of the induced roll magnetic separator to decrease the pyritic sulfur in coal freshly pulverized in a hammer mill, roll mill, and impact pulverization mill, indicates that these pulverizers do not increase the magnetic susceptibility of the pyrite particles to the magnitude necessary for magnetic separation.
An optical analysis of the distribution of pyrite in the coal samples revealed that 80 percent of the pyrite particles were either free or nearly free, that is, particles contained less than 20 percent coal by weight. Slightly more than 70 percent of the total pyrite particles were less than 44 µ, in size; of the free or nearly free particles 60 percent by weight had a size less than 44 µ. Early separations of minus 44 µ, pyrite in the absence of coal appeared to be successful. It was thought that crushing of pyrite in coal may not produce enough friction heat to cause surface reactions to an extent comparable to that caused by crushing pure pyrite. Therefore, a sample of hydrothermal Rico pyrite (0.3 x 10 -6 for 1/8-inch lumps) was crushed to less than 150 µ in an agate mortar. The susceptibilities of 150 to 44 µ, and minus 44 µ, fractions of the freshly ground samples were identical, that is, +0.3 x 10 -6, indicating no benefication from crushing.
Magnetic Susceptibility and Separation of Untreated Coals
Seven coals pulverized to minus 150 µ were separated using an induced roll magnetic separator. Each coal was passed three times through the separator under the same controlled conditions. The sulfate and pyritic sulfur contents of the original and final samples are given in table 7. Coal recovery upon three passes and the magnetic susceptibilities of the products are included in the table. Coal recovery ranged from 66 to 87 percent; in general, it was above 80 percent. The separator was set such that about 90 percent of the coal would fall into the lower susceptibility hopper upon a single pass. About 60 percent of the pyrite in two coals could be separated with yields of 66 and 75 percent. Two coals separated rather poorly, yielding 13 and 7 percent reductions in pyritic sulfur.
At the time these experiments were conducted, no examinations were made of the distributions of pyrite. It is very likely that size distribution and the state of liberation of pyrite had an influence on the results observed. However, from an inspection of the table, we also observe an influence of the sulfate sulfur. With the exception of Hocking No. 6 coal, a trend can be noted, that is, an increase in reduction of pyritic sulfur with increase in sulfate sulfur.
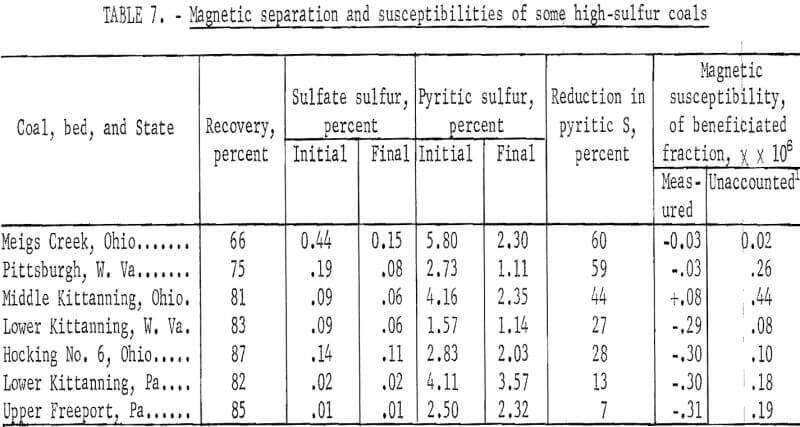
Of particular interest are the magnetic susceptibilities of beneficiated coals. With the exception of Middle Kittanning coal, all are diamagnetic; the diamagnetisms of four of them are very pronounced if we consider the fact that the magnetism of pure coals of the same rank is about minus 0.5 x 10 -6. The susceptibilities of the initial samples are not reported mainly because iron dust and other ferromagnetic contamination rendered them meaningless, that is, no reproducible results were obtained for carefully riffled samples. It was observed that in a magnetic separation such materials stick to the pole faces of the magnet. Therefore, in later studies, an initial scalping separation was made to remove ferromagnetic impurities. Such a separation removed less than 1 percent of the coal and the scalped product had a uniform susceptibility. In general the scalped samples show susceptibilities between 0 and 1 x 10 -6. For example, five different batches of Pittsburgh bed, W, Va., coal were measured after scalping and had magnetic susceptibilities of 0.40, 0.33, 0.49, 0.40, and 0.37 x 10 -6.
A knowledge of the pyritic and sulfate sulfur content enables the calculation of the combined susceptibility due to coal, pyrite, and melanterite. Such calculations yield susceptibilities slightly lower than those observed. The unaccounted susceptibilities are also included in table 7. From an inspection of tables 5 and 7, we note that only trace amounts of ferromagnetic iron must be present in the samples.
Magnetic Susceptibility and Separation After Treatment in a Fluidized Bed
A Pittsburgh bed coal was pulverized to minus 300 µ in a hammer mill. A scalping magnetic separation removed slightly less than 1 percent of the coal. The coal thus purified showed no reduction in sulfur content. The magnetic susceptibility of several riffled batches of the coal upon purification was remarkably uniform, that is, between 0 and 1.0 x 10 -6; the susceptibilities of the removed material varied from about 5 to 20 x 10 -6. Heat treatments were made in a 1-inch-diameter Pyrex tube, vertically placed in a furnace. The coal was supported by a fine screen. Below the screen the tube was packed with Pyrex wool to preheat the gas. A synthetic producer gas composed of CO, and N2, in the ratio of 20:20:60, was metered at a rate sufficient for a fluidization state at which the expanded volume was 2.5 times the packed volume of the solids. Treatment temperatures chosen were 250°, 300°, and 350° C and treatment times, 5 to 60 minutes. Upon the admission of coal, the desired temperature was reached within 5° C in less than 20 seconds.
The treated samples were separated in an isodynamic separator at identical free-fall rates at a setting (the same for all samples) to yield a recovery of about 90 percent. Magnetic separation of an untreated sample resulted in 27 percent reduction in pyritic sulfur. Treated samples resulted in reductions ranging from 32 to 45 percent with no consistent trend with treatment temperatures or time; thus, treatment was responsible for 5 to 18 percent additional reduction in pyritic sulfur.
The magnetic susceptibilities of beneficiated and magnetic fractions are shown in table 9. It is seen that the susceptibilities of the beneficiated fractions were remarkably uniform and mostly diamagnetic as in the case of separation using an induced roll magnetic separator. The susceptibilities of the magnetic fractions ranged from 0.8 to 3.3 x 10 -6. The fact that reasonable separation was attained at these low susceptibilities is very encouraging. In view of the fact that the susceptibilities of the material removed by scalping ranged from 5 to 20 x 10 -6, we may conclude that a magnetism of 2 to 10 x 10 -6 in a pyrite particle would be ample for its sufficient separation from coal. This level of magnetism requires the conversion of 0.1 percent of pyrite into a ferromagnetic form. Evidently such a conversion does not occur with heat treatment for a reasonable time at temperatures below 400° C,
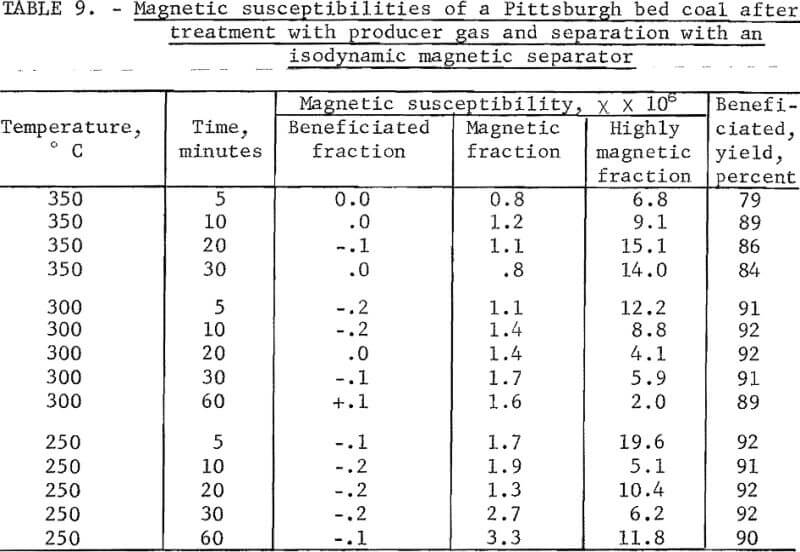
Although a second scalping was not made after heat treatment, the use of the isodynamic magnetic separator in the free-fall mode, resulted in an additional scalping for each separation. This is reported as the “highly magnetic fraction” in table 9. It consists of the 0.6 to 1.5 percent of the sample that clung to the pole pieces of the magnet. It was very difficult to remove from the pole pieces without losing part of the sample. This may partly explain its fluctuating magnetic susceptibility which shows little correlation with the temperature or time of heat treatment.
Summary of Results and Conclusions
The inherent susceptibilities of coals (about minus 0.5 x 10 -6) and of pyrite (0.3 x 10 -6) do not permit an effective separation of pyrite in a large-scale operation. Present experiments indicate that an effective susceptibility >3 x 10 -6 for pyrite would be sufficient. Therefore, only a small fraction of pyrite needs to be converted into more paramagnetic or ferromagnetic compounds of iron.
Paramagnetism of pyrite is not enhanced upon crushing. However, subsequent weathering or heat treatment increases its susceptibility. The increase appears to be mainly the result of formation of iron sulfates rather than of ferromagnetic compounds of iron. Upon weathering or heat treatment of coals, separation efficiency of pyrite increases with increase in sulfate sulfur content. The role of weathering was overlooked in previous studies and in early stages of the present studies.
For the pyrite to be separated, coal needs to be crushed to such a fineness that pyrite particles are free or nearly free. The required fineness depends upon the size and mode of distribution of pyrite in coal. This important factor also has not been well considered in previous studies. Conflicting results reported in the literature regarding the magnetic separation of pyrite from untreated or heat-treated coals are probably due to differences in the degree of weathering or in the pyrite size distribution.
Freshly ground coals are not beneficiated appreciably upon magnetic separation. Heat treatment at temperatures below 400° C improves separation to a limited extent. Higher heat-treatment temperatures are precluded mainly because of accompanying decomposition of coals. Sulfate formation by weathering is a slow process; storage for several weeks is required for a reasonable separation. The effectiveness of heat treatment diminishes with weathering.
It appears that effective beneficiation of coals can be achieved by magnetic separation. Results of previous studies and of some experiments reported here may suggest that the above conclusion should be made with some reservations. However, in the present studies the broad aspects of the problem received particular attention with the objective of narrowing the scope of future studies. The relative importance of the factors involved, especially that of size distribution of pyrite, became evident only in the later stages of the investigation. The degree of liberation of pyrite and the apparent susceptibility of pyrite in the treated coal are the most important parameters. Therefore, distribution of pyrite in coals and the necessary fine crushing to liberate it should be studied in great detail.
Very little attention has been paid to the magnetic separators. It appears that particle size as well as density influences the mode of operation of a separator. The development of a magnetic separator adaptable for large- scale operation requires further attention.
An integrated study should be made of the liberation of pyrite from coal, of increasing the pyrite susceptibility, and also of the method of magnetic separation for several coals with differing modes of pyrite distribution. The intervals of time between crushing, treatment, and separations should be limited and simulate an industrial operation.
Selective Heating of Pyrite in Coal
Introduction
From the preceding studies it becomes evident that pyrite is not converted into paramagnetic compounds of iron in any significant quantities by heat treatment below 400° C. Calculations readily show that most of the observed susceptibility of scalped coals, treated or untreated can be accounted for by those of pure coal (minus 0.5 x 10 -6), pure pyrite (0.3 x 10 -6), and ferrous sulfate (200 x 10 -6 per g Fe). The unaccounted magnetism is small (table 7), and the required concentration of ferromagnetic compounds to account for the unexplained or unaccounted susceptibility would be only a few ppm.
Decomposition reactions becomes detectable at temperatures well above 500° C and have high energies of activation. For example, at a temperature of 800° C the expected half-life of pyrite pyrrhotite conversion would be on the order of a second or less. It would appear that if pyrite were selectively heated to a high temperature, say >600° C, a sufficient fraction of it could be converted into ferromagnetic compounds of iron in a few seconds to render it highly paramagnetic.
Radiation Heating of Pyrite
For a verification of the preceding deduction a simple experiment was conducted. A 2.5 cm Pyrex tube, 30 cm long, was placed in a 3.5 cm Nichrome helix furnace, 15 cm long. The wire helix, having turns about 4 mm apart, was held together by cementing four thin ceramic bars on the wire longitudinally. The helix was heated to a deep red so that a thermocouple placed inside the Pyrex tube indicated a temperature approaching 700° C. The closely sized fractions of pyrite, less than 210 µ, were dropped through the Pyrex tube by means of a funnel. Regardless of size, the pyrite collected at the bottom had sufficient susceptibility to be removed by an ordinary magnet. The smaller particles, of course, became more strongly magnetic.
The fact that coal decomposes at temperatures above 400° C renders radiation heating not feasible. The only premise on which such a process may be considered is that the activation energy of decomposition of pyrite is much higher than that of decomposition of coal and hence sufficient magnetism may be imparted to pyrite with little loss in volatile matter of the. coal. However, radiation heating requires a very dilute phase and the experimental procedure may be very complicated.
Dielectric Properties of Coal and Pyrite
For absorbing materials the complex dielectric constant is expressed as
![]()
where ε is the real dielectric constant and σ is the electrical conductivity at the frequency υ; σ/υ is commonly referred to as the imaginary (part of) dielectric constant. In terms of refractive index n and extinction coefficient k, the real and imaginary dielectric constants are expressed as
ε = n² – k²…………………………………………………………(2)
σ/υ = nk……………………………………………………………(3)
The decrease in the energy density w of an electromagnetic wave upon traversing a distance t through a medium whose imaginary dielectric constant is nk is in accordance with the well-known relation,
Δw = wo[1 – exp (-4πkt/λ)]……………………………………(4)
λ being the wavelength, in vacuum, of the electromagnetic radiation, and wo the initial energy density.
If the imaginary dielectric constant at a frequency υ, υ = c/λ, of a material is negligible, the material is said to be transparent, nonabsorbing, or nonconducting. Consider an inhomogeneous material, made up of transparent and absorbing components. The electromagnetic radiation traversing it would be absorbed only by the conducting component, resulting in selective heating of the component. If pyrite in coal is to be selectively heated to a very high temperature, it is necessary to find a region of frequency, if any, at which the imaginary dielectric constant or conductivity of pyrite is much higher than that of coal. For this reason an investigation was made of the electrical conductivities of pyrite and coal in the microwave frequency region which is commonly utilized for dielectric or induction heating.
In the frequency range 50 khz to 60 Mhz, the measurements were made on a General Radio Type 160A Q-meter. The sample was placed between the plates of a capacitor which was in parallel with the Q-meter circuit. In order to get a reading for pyrite or coal, it was necessary to place a piece of plastic of the same size as the pyrite with the pyrite in the capacitor. Measurements were also made with plastic only in the capacitor in order to calculate the effect of the plastic on the reading of the pyrite and coal. A sample of coal and two samples of pyrite, one hydrothermal Rico pyrite and the other a coal pyrite, were utilized in the measurements. The results obtained are shown in figure 2. The wide scatter seen in the figure is due to low conductivity of the material studied. For some frequencies no conductivities were calculated because the readings were not sufficiently changed by inserting the capacitor with the sample into the circuit to enable a calculation to be made. The accuracy of several other measurements for both the pyrite and coal is questionable because of the necessary dividing by the difference of two measured quantities which are approximately equal. In particular, all results below 5 Mhz are of questionable accuracy. However, it is clear that in the 5 to 60 Mhz frequency range the electrical conductivity of pyrite is about one hundredfold that of coal.
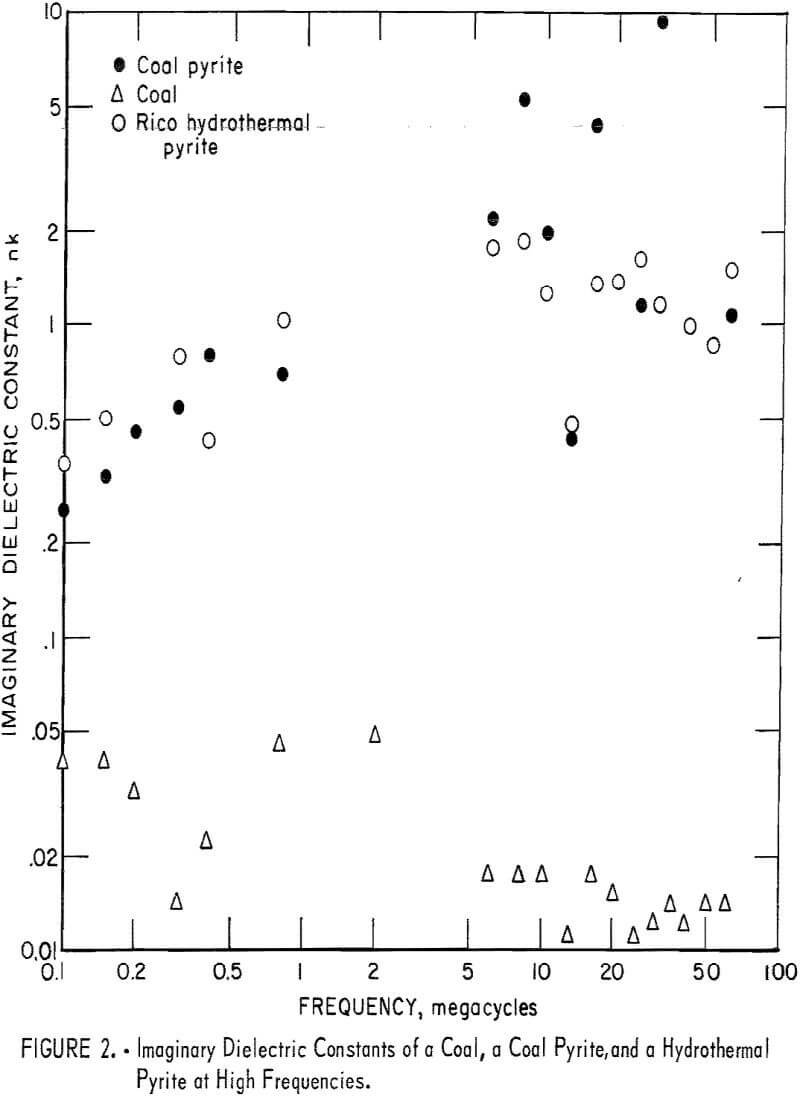
At υ = 30 Mhz(λ = 10³ cm) a particle of pyrite about 1 cm diameter would be required to absorb 1 percent of the radiation. If the dielectric constant continues to be constant up to about 30 Ghz, a 100 µ pyrite particle would absorb 1 percent of the incident radiation. Although some additional measurements were made using a General Radio Type 1607-A Transfer Function and Immitance Bridge up to 900 MC, it has not been possible to make systematic measurements extending into the Ghz region. An impression was gained that pyrite has an absorption peak in the Ghz frequency range.
Exploratory Studies of Dielectric and Induction Heating of Coals and Pyrite
Essentially measurements were confined to 30 Mhz, 2.45 Ghz, and X-band (10 Ghz). At 30 Mhz a commercial induction heating unit and dielectric heating unit were utilized. Samples of pulverized mineral matter from coal, coal pyrite of different size fractions and a coal containing about 5 percent pyrite were utilized. Samples were placed in Teflon tubes or trays. In every case coal did not become warm enough to feel by hand. Minus 140 µ, pyrite became warm but not magnetic. Coarse pyrite, about 1 mm, however, became magnetic enough to be attracted by a hand magnet. Coal containing pyrite was not affected.
A second series of experiments was conducted at a frequency 2.45 Ghz, using a Raytheon KV-104 microwave power generator with 125 watt output. The cavity of the generator was fitted with a Teflon tube. An assembly was made such that a continuous plastic belt could be moved at controlled rates through the tube. Inside the tube, the belt formed a trough. Powdered samples were dropped on the belt by means of a funnel. As before, mineral-matter-free coal was unaffected. Pyrite samples became hot enough to burn or deform the plastic belt. Collected samples were definitely magnetic. The results using coal containing pyrite were not conclusive; however, a systematic study required a unit having more power and construction of a heat-resistant nonconducting belt. Such studies were deferred pending the results of other exploratory studies.
Finally the cavity of an X-band generator unit was utilized for heating studies. In these experiments the temperatures of coal samples, a few millimeters thick, rose to 150° C upon exposure up to 30 minutes. Pyrites, however, regardless of their size, became heated to temperatures above 300° C in a few seconds; they became very magnetic.
