Table of Contents
An experimental procedure was developed for studying magnetic roasting on a accurately-controlled laboratory scale. The loss-in-weight method was used and the course of reduction could be followed under a reducing gas atmosphere containing as many as five different components. Some important variables of the operation were checked on a marginal Mesabi iron ore containing porous and dense varieties of hematite as well as pure goethite.
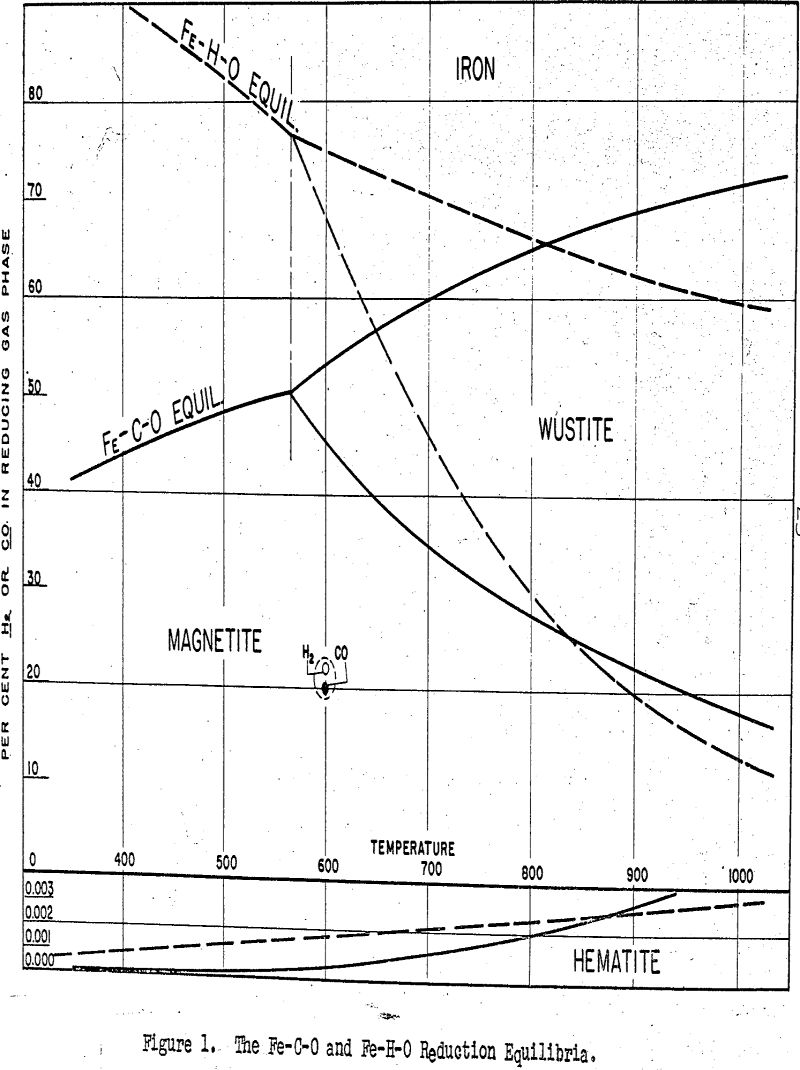
The conditions simulated were those for the grate roasting of a coarsely-sized ore burden. Temperature was maintained at 600°C; gas composition at 4.25% CO, 4.25% H2, 17.0% CO2, 15.0% H2O, and 59.5% N2; and gas flow rate at about 15 lineal feet per minute. The ore charge was composed of quarter-inch cubes.
Experimental Roasting Conditions
The initial laboratory conditions for the present investigation were stipulated by the Staff of the Mines Experiment Station and so chosen as to simulate actual pilot plant practice being used on Mesabi Range material. The conditions were as follows:
- Temperature………………………………………………………600°C (1650°F)
- Sample size…………………………………………….approximately one-fourth
inch cubes. The pilot plant test batches were being run on minus one-half inch material, much of which was in a striated and layered condition. Any trouble developing during the reduction roast would undoubtedly be related to the larger pieces of the denser ore fractions.
The gas phase being used in the pilot plant work was formed by the partial combustion of natural gas. Five gaseous components were possible and the desired gas analysis was set at:
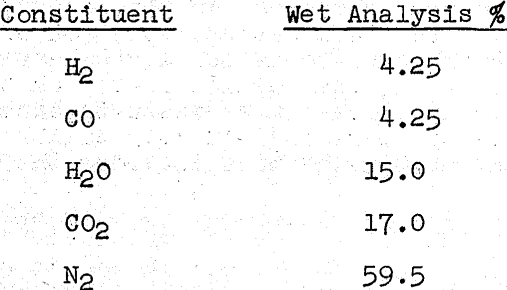
Information on the required flow rate for reduction roasting is quite scarce. Some figures have been quoted for commercial practice and range from about 50 to 100 feet/minute on a lineal flow rate basis. To check these figures, a series of runs was made on a laboratory scale in which this variable was studied, and it was found that a rate of 1000 cm³(SC)/minute was sufficient to minimize any flow rate effects.
From the conditions being listed, a gas stream containing five components (H2, CO, H2O, CO2 and N2) in the noted proportions and flowing at a rate or 1000 cm³ (SC)/min. was a prime requisite of the investigation. Since the amount of gas needed was small, it was inconvenient to try and prepare this mixture by the commercial method of partially combusting natural gas.
The condensible component, H2O, posed other experimental difficulties, for at the concentration desired (15 per cent), a heated system was necessary to generate and transport the component from its source to the reduction furnace proper.
Experimental Procedure
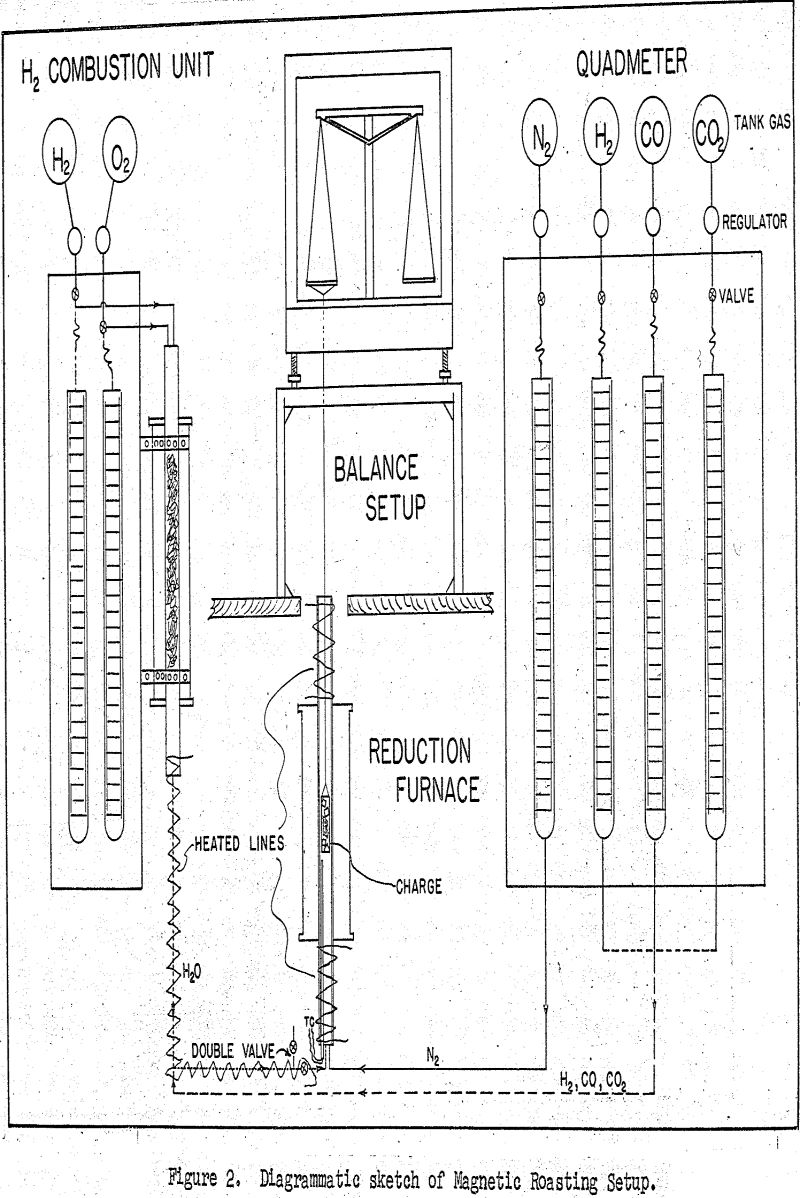
The heart of the setup is the vertical resistance furnace which was equipped with a one inch quartz combustion tube for carrying out the reduction reactions under controlled atmosphere. This reduction furnace was controlled electrically by a Celectray controller capable of maintaining the reaction temperature to within ±1°C.
For following the rate of reduction, the loss-in-weight method was used and the reduction charge was held in a nichrome gauze basket which was in turn suspended at the center of the reduction furnace and continuously weighed by an overhead analytical balance.
A typical run was started by suspending the charge at the center of the furnace and heating it to the reaction temperature of 600°C under a nitrogen atmosphere. This nitrogen was actually the nitrogen component of the forthcoming gaseous reduction mixture and was metered by the nitrogen flowmeter of the quadmeter bank at the delivery rate of 595 cm³(SC)/minute. As the reduction charge was coming to thermal equilibrium, the hydrogen combustion unit was adjusted to deliver the requisite amount of water vapor at the rate of 150 cm³(SC)/minute. When the run was ready to commence, this same gas mixture was diverted instantly into the reduction chamber to join the nitrogen component and thus to start the reduction under the full required flow of 1000 cm³(SC)/minute.
Marginal Ore Considerations
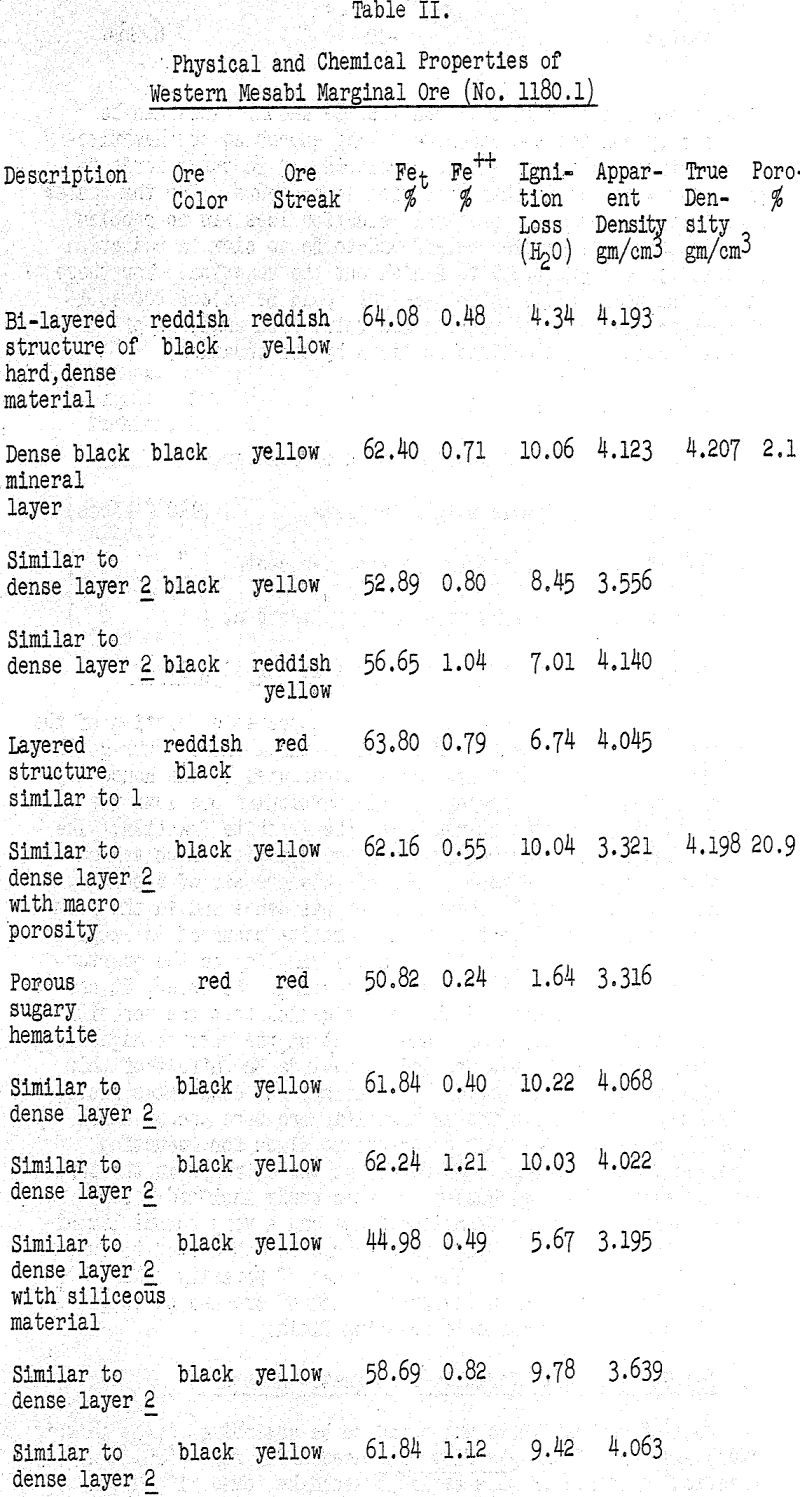
The preliminary studies on magnetic roasting at the Mines Experiment Station were being conducted on a batch of Western Mesabi marginal ore designated as Batch No. 1180.1. This ore was screened to a minus one-half inch size, and some trouble was being experienced in attaining a complete magnetic roast of the ore under the conditions previously listed.
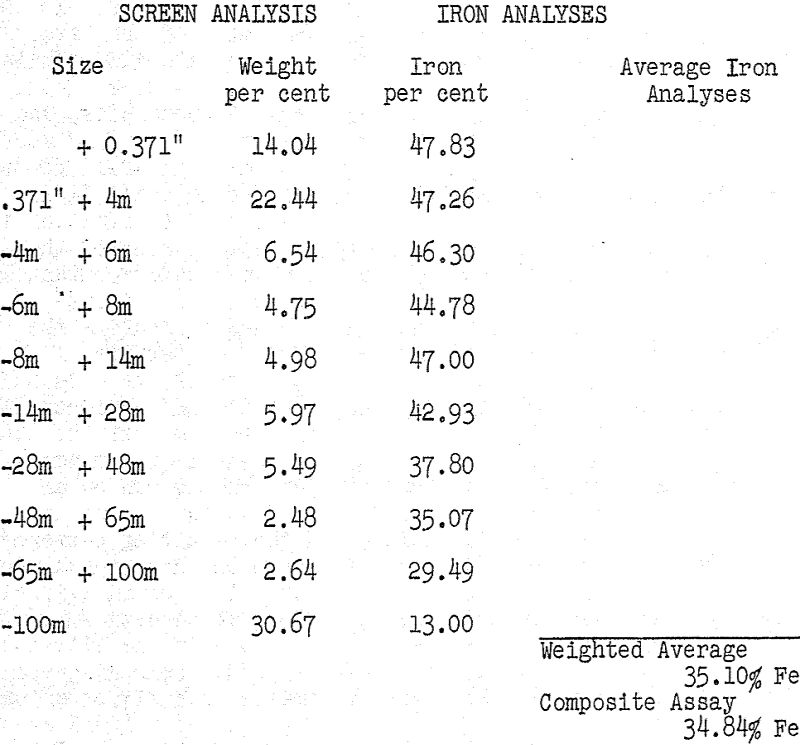
The first approach to the problem was an examination of the ore itself and particularly the screening and analytical data which were available on the minus half-inch material being processed. The information is summarized in Table I and it is immediately apparent that the larger particles were also the richer ones with regard to iron content.
To arrive at a better understanding of the ore structures involved, recourse was made to the original stock pile from which the half-inch charge material had been derived. About 200 pounds of plus one inch material was screened from the pile and washed thoroughly. A further cut was made by hand-picking out the larger and denser-appearing chunks to produce a working sample of about 50 pounds. Macro-examination of this lot was accomplished by sectioning 100 random pieces on a carborundum cut-off wheel.
Reduction Studies
The experimental procedure for the magnetic roasting studies has been described in a previous section. Ten one-quarter inch cubes were used for each run and the reduction progress of each charge was followed by measuring its loss-in-weight as a function of time. To put the loss-in-weight data on a per cent basis, the
total reduction loss in each run must be known. This can be assessed by running the experiment long enough so the hematite-to-magnetite transformation is completed, or in other words to the point where no further reduction loss occurs. For the faster runs this determination of total reduction loss was no problem.
It has already been shown that an appreciable fraction of the ore lot under investigation (No. 1180.1) was a fairly pure goethite material whose properties were well represented by the dense layer of MEX lump no. 2. Accordingly, this particular ore lump was chosen as the reduction standard for the goethite fraction. The rest of the ore lot was mostly low-grade hematite which varied greatly in physical texture. Some of this ore was of a porous, sugary consistency while the remainder was dense and in the form of thin layers.
Since reduction of the ore takes place at 600°C, the reducing gas interacts with material that is in the completely dehydrated state. With normal hematitic ores, very little dehydration takes place and the porosity In the 110°C dried condition is about the same as in the 600°c “dehydrated” condition. With limonitic and goethitie ores, however, the removal of from 6 to 10 per cent water of crystallization may have an important effect in increasing the porosity of the ore.
The dehydration of the various ores was accompanied by some rather unusual behaviour patterns. For instance, the table shows an interesting correlation concerning the volume change in the ore brought about by the dehydration process.
To correlate the porosity function with the reduction data, the “time for 90 per cent reduction” for each of the three ores is plotted against its respective porosity in the dehydrated state. The resulting curve shows the marked change in reduction rate afforded by the porosity variable and also sets a rough limit as to the porosity desired in larger pieces of ore when subjected to reduction roasting.