During the past few years a radically new process for the magnetic roasting of iron ores has been investigated and developed by Pickands Mather & Co. and the Erie Mining Co. in the Erie laboratory at Hibbing, Minn. This process, originally devised by Dr. P. H. Royster of Washington, D. C., involves the use of a roasting technique quite different from older methods. It has now been demonstrated that iron-bearing materials can be roasted as effectively as by any previously known method, and at a much lower cost.
Heretofore the basic concept behind most magnetic roasting processes has been the idea of heating iron ore to a temperature of 800° to 1100°F in a strong reducing atmosphere, preferably either carbon monoxide or hydrogen. Temperatures under 800 °F were undesirable since excessive roasting time was required. Temperatures over 1100 °F were avoided because of the danger of converting part of the iron to ferrous oxide which is nonmagnetic.
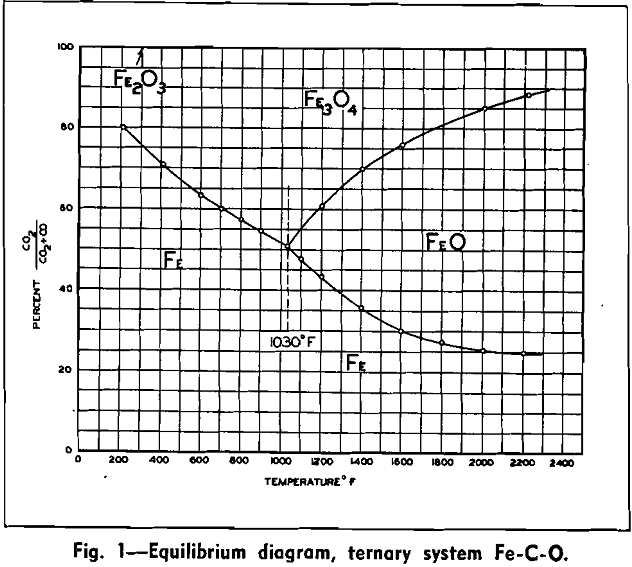
The theory of controlled atmosphere during the roasting operation can best be appreciated by inspecting the equilibrium diagram of the Fe-C-O system. An inspection of this diagram shows that in certain areas magnetite, Fe3O4 is the only stable form of iron. A further inspection of this table shows that if the proper ratio is maintained between carbon dioxide to carbon monoxide, such a gas will be reducing with respect to hematite, Fe2O3 and will be oxidizing with respect to both ferrous oxide, FeO, and iron, Fe. It should be kept in mind that the formation of ferrous oxide in a roasting operation is harmful, since this oxide is nonmagnetic; if it forms in any quantity, it will cause substantial loss of iron in the ensuing magnetic separation step. If a ratio of approximately three parts carbon dioxide to one of carbon monoxide is maintained, the resulting operation can be carried on at a relatively high temperature without fear of over-reduction.
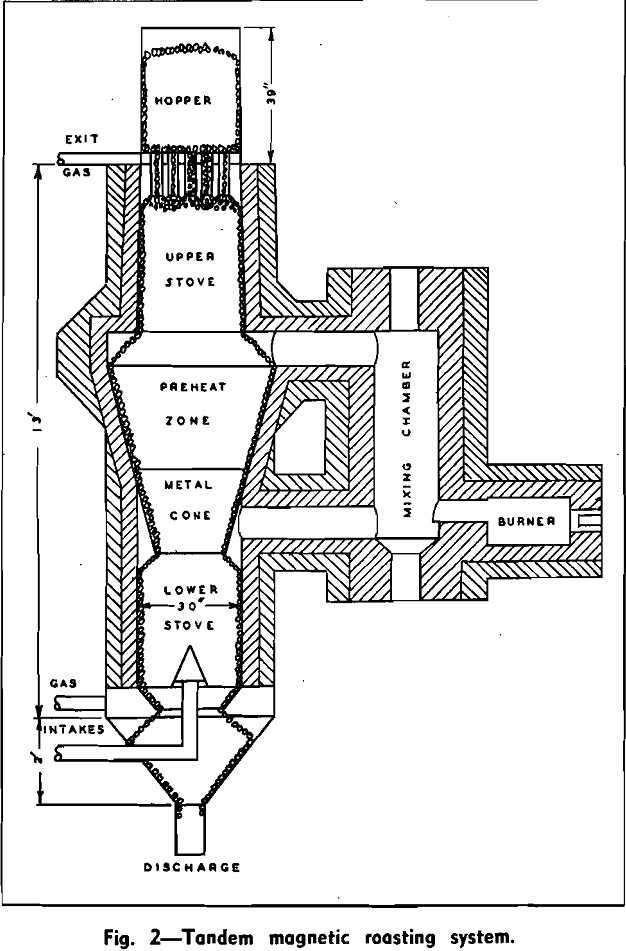
This furnace is circular and has an ID of 2½ ft. It is capable of roasting from 1 to 1¼ long tons of iron ore per hr. In practice, the furnace is charged with crushed ore and is fed through either a double bell arrangement such as is used on blast furnaces or by the hopper-like device shown. The shaft of the furnace is filled completely with ore, which moves down the shaft as the material is discharged from the bottom. Basically, the furnace consists of an upper and lower stove with a middle chamber in between. A mixing chamber and combustion chamber are also provided. An optional furnace design is to have two stoves in parallel and operate them intermittently, as in the manner of a pebble heater. Since two stoves are superimposed one upon the other in the present installation, it is called a tandem furnace. In the upper stove, the cold moist ore is heated up to the desired temperature by uprising gases. The gases are of such composition, to be discussed later, that the descending ore is reduced and the iron oxides converted to magnetite. Ore from the upper chamber travels through the middle zone of the furnace where the area of discharge into the lower stove may be restricted. Weak reducing gas is blown up through the lower stove, cooling the hot descending roasted ore.
The gases blown through the furnace serve a two-fold purpose, to reduce the iron oxides and also to act as a carrier gas or heat exchange media. For a carrier gas, the amounts must be closely regulated so that sufficient cooling gas is supplied at the bottom of the furnace to abstract the heat from the discharged ore. Likewise, the volume of heated gas must be such that it supplies the necessary heat to the raw ore fed to the furnace.
The basic reducing reaction that takes place in the roasting zone is: 3Fe2O3 + CO to 2Fe3O4 + CO2.
This reaction is exothermic and liberates 88 Btu’s per pound of magnetite formed. This heat is liberated in the upper stove and contributes substantially to the heat economy of the system. The mass of ore in the constricted area in the middle stove serves a useful purpose other than a heat recuperator in that it insures that a greater portion of the gas from the lower stove will pass through the mixing chamber and be brought up to the desired temperature. This permits a close control of the temperatures in the roasting zone.
During the past five years, in the development of the process, many low grade iron ores from the Lake Superior district have been roasted. Such materials as oxidized taconite and low grade ores from the Minnesota, Wisconsin, and Michigan ranges have been tested. In general, the optimum roasting technique, as far as temperature of roast and gas composition are concerned, is similar for all.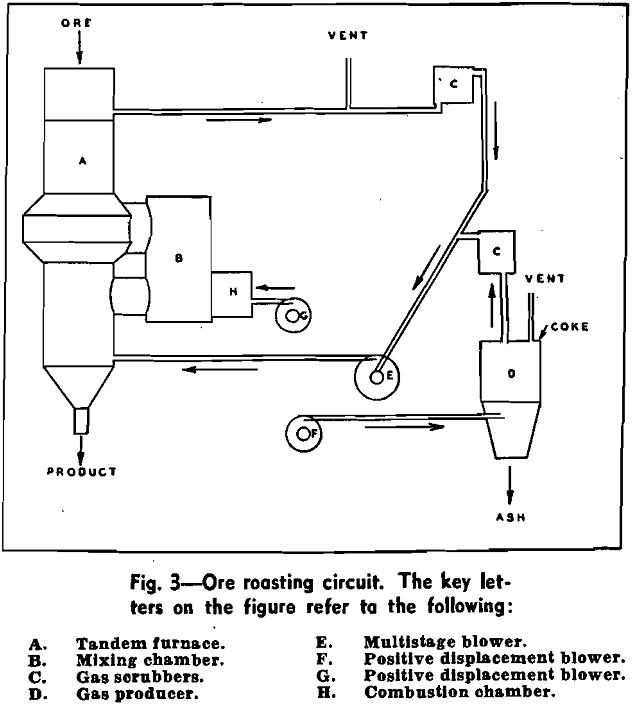
The overall iron unit recovery was 90.63 pct. It should be remarked in passing that the magnetic roasting step, in addition to putting the ore into a form that can be readily and cheaply concentrated, usually makes it much more amenable to grinding. The power required to grind a roasted taconite is about half that needed to grind unroasted taconite.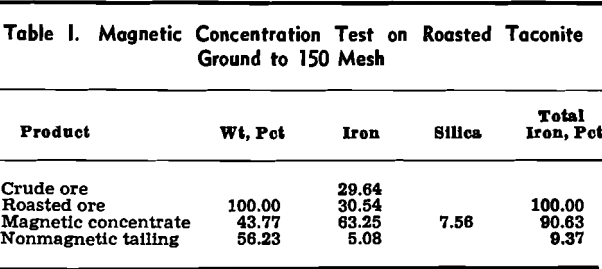
With most ores, the solution of the fines problem is to screen and then briquette the fines with a cheap binder, such as waste sulphite liquor, and charge these briquettes into the furnace with the coarse material. Such briquettes hold together well during the roasting operation and are sufficiently porous so that the conversion to magnetite is excellent.

