Table of Contents
The concurrent observation of the phenomena of alkali-aggregate reaction and development of efficient dust collection systems on rotary-kilns made mandatory the process modifications to produce low alkali cements. Such low alkali cements as were available prior to these recent changes were either the rare result of low alkali raw materials, the loss of alkalies, with dust loss from the kiln stack, or in some instances by the use of CaCl2 in the kiln feed to increase volitalization of the alkalies and their subsequent loss or discard with the normal dust emission from the kiln.
The Alkali-Aggregate Problem
It is only recently that alkali-aggregate reaction phenomena have been identified, studies and conclusions reached that low alkali, (below 0.6% calculated as Na2O), cement would in most cases eliminate or reduce the distress observed in concrete by reason of reaction of the alkali content of the cement and various forms of amorphous silica.
More, recently deleterious reactions have been, observed and substantiated between the alkali content of cement and dolomite aggregates.
The alkali-aggregate reactions are most frequently reported in areas of the North American continent with few authenticated cases of troubles arising from this reaction in Europe. In one of the most recent publications on portland cement by Czernin the statement is made that “There is little danger of concrete damage due to alkali-aggregate reaction with the aggregates normally used in the United Kingdom or in Europe”.
The Influence of Dust Collection Systems
Concurrently with awareness of the potential problems of alkali-aggregate reaction came the installation of efficient electrostatic or glass bag dust collectors. Imposed upon cement manufacturers by newly established air pollution control laws and/or the economics of wasting variable percentages of raw material through stack losses, the installation of these dust collecting systems captured the alkalies, volitilized in the kiln burning zone and previously lost to the surrounding countryside.
Thus with the alkali-aggregate reaction knowledge came the recovery of more alkalies, their reintroduction into the kiln and the subsequent higher alkali content of the product. An example of this condition was readily observed at the Kansas City plant of Missouri Portland Cement Company where kilns operating with no dust collection averaged from 0.6 – 0.75% alkalies as Na2O; to kilns with electrostatic dust collection and return resulting in a alkali content of 0.8% to a maximum frequently as high as 1% as Na2O.
Wet Process Leaching Systems
The operation of dust leaching systems on wet process kilns has been successfully practiced for the past several years. In essence the collected dust is mixed with at least ten times its weight with fresh water, dewatered usually by a small thickener and pumped back into the kiln along with new feed. Thickener underflow will normally be at 1 part dust, and 1 part water, the remaining 9 parts of water removed by thickener overflow containing the soluble alkalies and sulfates is wasted to sewer.
As reported in these previous papers, difficulties are encountered in maintenance of the thickeners, elimination of scaling in the pipe lines, and buildups of hydrated dust slurries at any quiescent location. These problems still exist, though some progress is to be reported on their solution. As more industry members have experienced the problems newer process changes have been instituted to alleviate trouble points.
While large volumes, of water are a prerequisite to operation of a dust leaching system, disposal of the alkali bearing waste water has in part been simplified by a neutralizing process. An ejector pumps a portion of the carbon dioxide bearing stack gases through the effluent water neutralizing the alkalies and producing, an acceptable waste water. In another installation, the dust is originally collected by a wet scrubbing system, subsequently dewatered and returned to kiln, the effluent from the scrubber is effectively neutralized by contact with the kiln gases.
Operational features have also helped reduce the problems that so frequently played early operations. By careful attention to pulp levels in the thickener and maintaining the level at the minimum allowable consistent with desirable water content of the underflow less frequent buildups occur on the rake arms.
Dry Process Leaching Systems
Much of the work done on wet process leaching systems could be directly transferred to dry process kiln installations merely sizing the equipment for the anticipated greater dust loading.
In normal wet process installations with efficient dust collection, the collected dust will range between a minimum of 2% by weight of the raw feed to a maximum of 8%. In a dry process kiln with equal efficiency of dust collection this collection of dust ranges from a minimum of 5% to a maximum of as much as 40%.
The percentage of dust throwoff from the dry process kiln relates in large part to kiln gas velocity, though it may be further influenced by configuration of internal heat exchangers such as trefoils, quadrants or chain systems.
With present inability to prehydrate kiln dust, the uneconomic nature of CaCl2 process, the uniform distribution of alkalies in both fine and coarse fractions of the kiln dust, a conventional dust leaching system was installed on two 350′ dry process kilns. With little alteration from the original systems on wet kilns (45′ thickeners in place of 28′ thickeners) the process was started.
Kiln Conditions and Their Effect on Dust Leaching
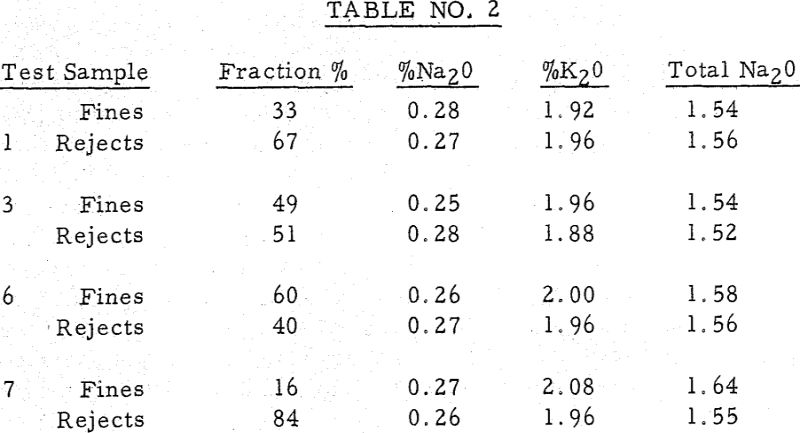
It was observed that despite successful operation of the leaching system and return of water soluble free alkali dust to the kiln that frequently low alkali cement did not result. It was apparent that the high percentage of raw feed in the dust with its unreacted alkalies did not successfully leach, and that even partially calcined materials did not result in sufficient alkali reduction by leaching.
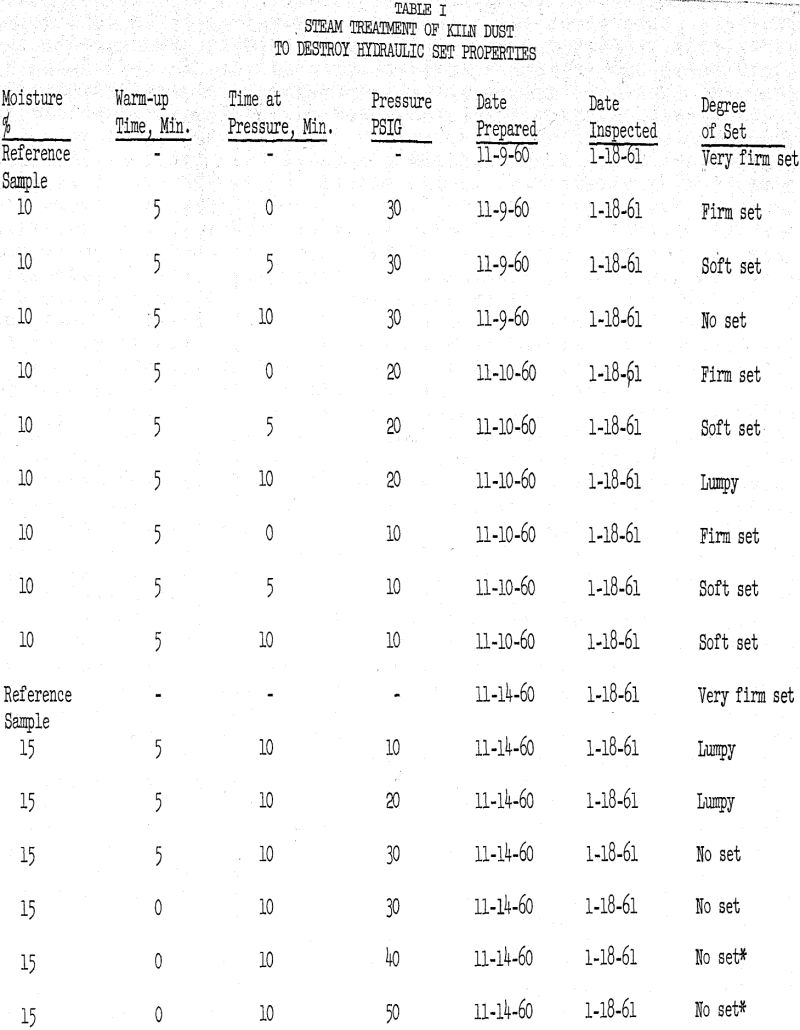
It had been predicted that if such a condition did exist that minor amounts of calcium chloride would react with the alkali minerals and create soluble forms. Under conditions of this nature, the addition of from one to two pounds per barrel of CaCl2 to the raw feed did, in fact, increase the solubility of the alkalies in the dust and resulted in low alkali cement.