Table of Contents
In late 1965 General Mills announced the availability of a new liquid ion exchange reagent LIX-64, for the recovery of copper from various solutions, such as dump leach liquors. Acceptance of this material by the copper industry has been gratifying. A number of pilot plants have been operated, and at this writing there are four commercial plants either in operation or under construction. The properties and operating characteristics of this reagent have been described previous papers.
Chemistry of LIX-64N
As with LIX-64, LIX-64N operates on a hydrogen ion cycle:
Extraction
(2RH)ORG + (Cu++ + SO4 =)AQ (R2Cu)ORG + (2H+ + SO4 = )AQ
Stripping
(R2 Cu)ORG + (2H+ + SO4 =)AQ (2RH)ORG + (Cu++ + SO4 =)AQ
Chemically, the two reagents perform similarly, except LIX-64N operates either in an extended pH range or will perform the same task in a reduced number of stages.
Laboratory Investigations
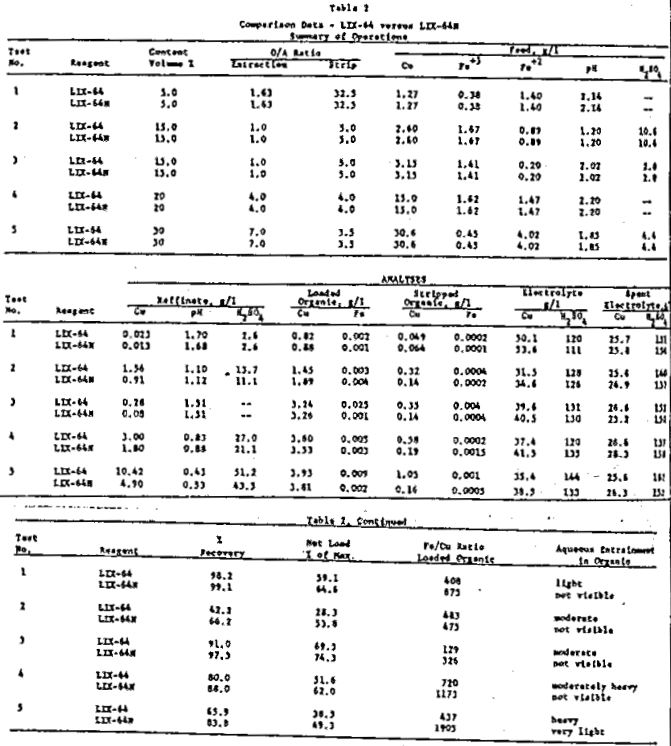
A pilot circuit was assembled in the laboratory, consisting of four extraction stages, three strip stages, and a small electrowinning cell. This circuit was operated with both LIX-64 and LIX-64N under conditions as closely identical as possible for comparison purposes. A number of typical acid liquors (obtained from actual southwestern copper operations) were used as feed solutions. These liquors ranged between 1 g/l and 30 g/l copper, with acid contents between 1.0 and 10.0 g/l H2SO4. Stripping was accomplished with a spent electrolyte containing about 25 g/l copper and 150 g/l free H2SO4.
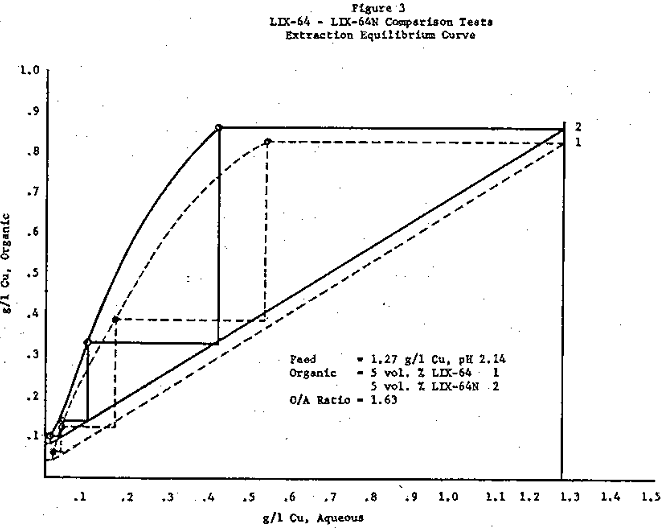
Feed for Test 1 contained 1.27 g/l copper, 0.42 g/l Fe+³, 1.56 g/l Fe+², at pH 2.14. The LIX content for the tests was five volume percent. O/A ratio in extraction was 1.63 and in strip was 32.5. LIX-64N produced a raffinate of 0.013 g/l copper for a recovery of 99.1 percent. LIX-64 under the same conditions produced a raffinate containing 0.023 g/l copper for a recovery of 98.2 percent. Iron rejection by the organic was improved with LIX-64N; rejection ratios were (Cu/Fe ratio, loaded organic): LIX-64N – 675, LIX-64 – 408. Net loading of copper on the organic was also better with LIX-64N. Data obtained showed (loading obtained/maximum loading): LIX-64N – 64.6 percent, LIX-64 – 59.1 percent. Visually, entrainment of aqueous in the organic was light with LIX-64, and not visible with LIX-64N.
Feed for Test 2 was 2.60 g/l copper, 1.67 g/l Fe+³, 0.89 g/l Fe+² at pH 1.20 (10.6 g/l H2SO4). LIX content was 15 volume percent. O/A ratios were: extraction – 1.0, strip – 5.0. Again, LIX-64N produced a considerably better raffinate, being 0.91 g/l copper for a recovery of 66.2 percent as opposed to 1.56 g/l copper at a recovery of 42.2 percent for LIX-64. Iron rejection ratios (Cu/Fe ratio, loaded organic) for the reagents were: LIX-64N – 475, LIX-64 – 483. Net loadings on the two reagents were: LIX-64N – 53.8 percent, LIX-64 – 28.3 percent. Entrainment of aqueous in the organic was moderate with LIX-64, and not visible with LIX-64N.
Physical Characteristics of LIX-64 and LIX-64N
Phase separation rates were determined for both LIX-64 and LIX-64N. Operations were conducted in a single mixer-settler stage at a fixed flow rate. O/A ratio for the tests was held at 1.0 and the continuous phase was organic. The settler was arranged in such a manner that the cross sectional area could be increased or decreased at will. The organic was contacted in the mixer with raffinate from test 3 above, and the system was allowed to stabilize. Measurement of the dynamic dispersion band width in the settler was taken, and the settler area was decreased. This procedure was continued until the settler flooded. Such tests were run at LIX reagent contents of 10, 20 and 30 volume percent for LIX-64, and at 10, 20, 30, 40 and 50 volume percent for LIX-64N. Temperature during all tests was held constant at 23°C (laboratory ambient). While General Mills realizes the phase disengagement rates will vary with temperature, it was the purpose of this investigation to determine only comparative data for LIX-64 and LIX-64N.
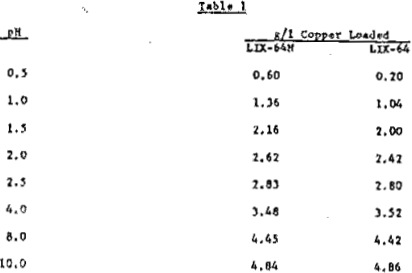
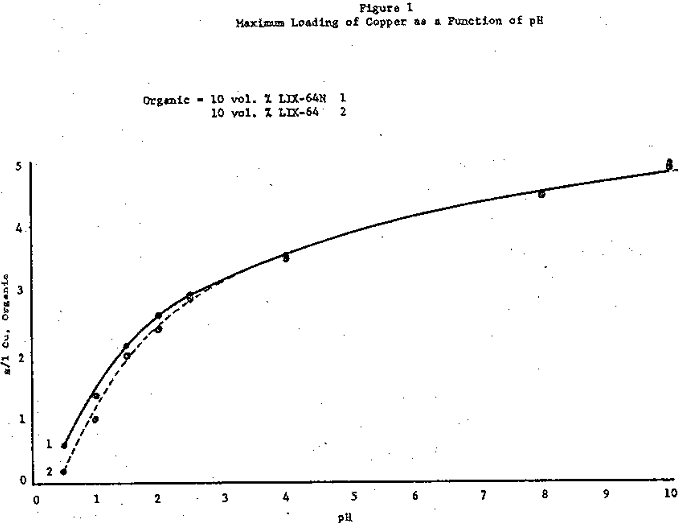
g/l Cu (organic)/0.25 = Percent LIX-64N