Table of Contents
The group of sulphates correspond to sulphuric acid, H2SO4. In the list of sulphate minerals, there are metals in place of the hydrogen of the acid; they are to be distinguished from sulphides, which contain no oxygen, but are made up of sulphur and metals.
Barite or Barytes
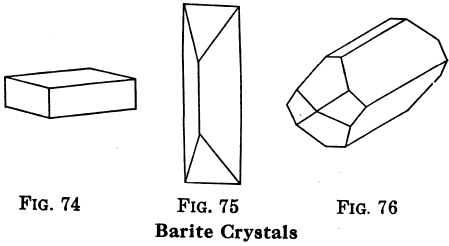
Barite or Barytes; BaSO4. — Also called heavy spar. Color, usually white, sometimes gray, reddish, brown, etc.; powder, white; luster, glassy; H = 2.5 to 3.5; G = 4.3 to 4.6; crystals in orthorhombic system, usually tabular, often in clusters with wedge-like edges projecting; cleavage, perfect, cleavage pieces with two sets of square edges and one set of 78° and 102° (Fig. 74.).
Found in veins in rocks of many ages and kinds, often along with ores of lead, copper, silver, etc., and with fluorspar. When ground to a fine powder, used in paper, linoleum, rubber, etc.; also, to make lithopone and other substitutes for white lead. Barite is the raw material for the manufacture of a number of chemicals, including barium nitrate, for green fire, but witherite is more easily worked for this purpose. Derbyshire spar is a dark brown variety found as stalactites, and used as an ornamental stone, for vases, etc. (See also Fluorspar) When ground, it sells according to purity.
Celestite
Celestite; SrSO4. — Color, white, often bluish and sometimes reddish; powder, white; luster glassy; H = 3 to 3.5; G = 3.95 to 3.97; crystals, similar to those of barite; cleavage, perfect, cleavage pieces with square edges (see Barite); composition, strontium sulphate. Celestite is often fibrous. Used chiefly to make strontium nitrate, for red fire, and strontium hydrate, for use in refining beet sugar. (See Strontianite).
Anglesite
Anglesite; PbSO4.—Similar to barite and celestite, but rather softer and much heavier; H = 2.75 to 3; G = 6.3 to 6.4; composition, lead sulphate. Found with galena as a result of its weathering. (See also Cerussite).
Gypsum
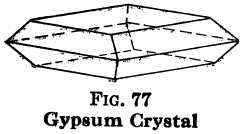
Gypsum; CaSO4·2H2O. — Color, white when pure, but variously colored with impurities; powder, white; luster, glistening, sometimes dull; H = 2; G = 2.3; crystals in monoclinic system; cleavage, very perfect, almost like mica in yielding thin, smooth leaves when crystals are large enough, but differs from mica in that the leaves will not return to original shape when bent (inelastic); composition, calcium sulphate (sulphate of lime), with water; slightly soluble in water.
Found in beds as a sedimentary deposit, often with rock salt, suggesting its origin by the evaporation of sea water; sometimes in crystals in clay and soil.
Selenite is gypsum in large, clear crystals, often very beautiful. Satin Spar is fine fibrous gypsum, with pearly opalescence. Alabaster is a fine grained, compact variety, white or of delicate shades.
Gypsum is used chiefly for making plaster of Paris, most of the water being driven off by heating in kilns, and then grinding. When plaster of Paris is mixed with water, the crystals of gypsum are again formed, and the whole mass “sets,” becoming solid. The ease with which gypsum is thus manufactured in any desired shape, its softness, and the heat and sound insulating qualities, make gypsum a valuable material for building construction. Gypsum is also used as a soil fertilizer. Clear crystals of selenite are used in optical instruments; satin spar is used for making beads and other ornaments; and alabaster is used for ornamental sculpture for monuments, vases, etc.
Anhydrite CaSO4, is often found with gypsum; it is of little value, but in exceptional cases is used to make sulphuric acid.
Epsomite or Epsom Salt
Epsomite or Epsom Salt: MgSO4.7H2O.—Appearance and taste are well known; H = 2 to 2.5; G = 1.751. Found in mineral waters and in deposits from alkaline lakes in British Columbia. Used in tanning, dyeing, for weighting silk, paper, and leather, in enameling, in fireproofing compounds, in medicine, etc.
Mirabilite or Glauber’s Salt
Mirabilite or Glauber’s Salt; Na2SO4· 10H2O.—A white easily soluble substance of a cool, slightly bitter taste, found as a deposit or incrustation around mineral springs, and in salt lakes. It may be of value to replace salt cake (Na2SO4) in the manufacture of pulp by the sulphate process.
Wolframite, or Wolfram
Wolframite, or Wolfram. — An ore of tungsten ; color, dark grayish or brownish black; powder, nearly black or brownish red; luster, somewhat metallic; H = 5 to 5.5; G = 7.2 to 7.5; cleavage, very perfect; sometimes weakly magnetic; composition, tungstate of iron and manganese, FeWO4 and MnWO4, containing about 75% of tungsten oxide, WO3. Huebnerite, is higher in manganese than wolframite, but much like it. Ferberite is essentially FeWO4. It is a heavy black mineral.
Found in granite and other acid rocks, often with tinstone; also, in quartz veins, and sometimes with gold. Used mainly for making tungsten steel, tungsten filaments for electric lights, and to make tungsten as an ingredient of stellite (see Chromite). It has also been used to make a “contact” material to promote the union of hydrogen and nitrogen to make ammonia, and in the manufacture of acetic acid. If the ore or concentrates carry say, 60% of WO3, or 1200 pounds in a ton of ore, it will sell well.
Scheelite
Scheelite; CaWO4. — Color, white, yellowish, brownish, greenish, reddish; powder, white; luster, glassy; H = 4.5 to 5; G = 6; cleavage, distinct; composition, calcium tungstate, or tungstate of lime. Usually found in quartz veins, sometimes associated with gold (as in the Porcupine area, Ontario), tinstone, wolframite, etc. It has also been found in contact deposits near intrusions of granite. See Wolframite.
