Table of Contents
Here is a List Of Native Elements Minerals & Naturally Occurring Metals In Pure Form are subdivided into two classes, metals and nonmetals, to accord with similar divisions in chemistry.
Gold
Symbol, Au.; color, yellow, but paler when mixed with silver, which is usually present in native gold; H = 2.5 to 3; G = 19.33 for pure gold, but is less in proportion to the amount of silver present, and it may be as low as 15. Malleable, and thus spreads or draws out without powdering when pressed with point of a knife. Electrum is a natural alloy of gold and silver; it is white or quite pale yellow. Pyrite and copper pyrites, which resemble gold in color, powder and turn black, while yellow scales of mica powder and turn white. Another distinction between these substances and gold is that a speck of gold shows the same brightness at every angle, but a bit of pyrite or yellow mica changes in brightness as it is turned. When the specimen is wet, the color of the gold comes out better. (Try this carefully with the specimens of gold, pyrite, etc.) Gold was the old-time standard of values (23.22 grains of pure gold being exactly equivalent to a dollar), and an ounce Troy was worth. At present, however, gold is worth more than its old standard value, and it is uncertain if it will ever again be used as the international standard for national currencies.
Gold is usually found in quartz veins in certain schists (schists are rocks having a sheeted or laminated structure) as chlorite, talc, and slaty schists; also in mica schist, hornblende schist, gneiss, diorite, porphyry, rhyolite, and occasionally in granite; and in South Africa, in an altered conglomerate. It likewise often extends into the wall rocks of such veins. Gold is commonly associated with pyrite, and a part of the gold is often in the pyrite. Other minerals found in gold veins are copper pyrites, galena, zinc blende, mispickel, tellurides, native bismuth, bismuthinite, native arsenic, stibnite, magnetite, hematite; also, sometimes, scheelite, siderite, fluorite, and apatite. In gold regions, gold is also found in the sand and gravel that is formed by the washing down of the weathered rocks and gold veins; this is known as placer or alluvial gold, and it is accompanied by such hard, heavy minerals as magnetite, garnet, zircon, topaz, corundum, etc., and sometimes by platinum.
Silver
Symbol, Ag; color, white on fresh surfaces, but tarnishes to brown or black; H=2.5 to 3; G = 10.5, but may be as high as 11.1 on account of gold (up to 10%) alloyed with it. When pressed with the point of a knife, it spreads or flattens, but does not powder. Silvery specks of galena or mispickel, which resemble silver, are powdered and turn gray or black; white silvery scales of mica, sericite, or talc are powdered and turn white. The value of silver varies from 60 cents (or sometimes less) an ounce Troy up to $1.00 or more (exceptionally). Found in veins in gneiss, schist, porphyry, etc.; in Ontario, in calcite veins in conglomerate, slate, and diabase.
Copper
Symbol, Cu; color, red but may be covered with black, brick-red, green or blue tarnish; H = 2.5 to 3; G = 8.8 to 9. Its malleability distinguishes it from other coppery-red minerals, such as niccolite and breithauptite, which are powdered black when rubbed with the point of a knife.
The value of copper when ready for the market has ranged in recent years from 25 to 40 cents a pound, but varies according to market conditions.
Native copper accompanies other copper ores, especially cuprite, malachite, and azurite. In the Lake Superior district, it is found in amygdaloid trap and sandstone, near the contact.
Platinum
Symbol Pt; color, light steel- gray; H = 4 to 4.5; G = 14 to 19; for the purified metal, G = 21 to 22. Native platinum is more or less alloyed, with iron (up to 18%), copper, palladium rhodium, iridium, and osmium. Palladium, rhodium, ruthenium, osmium, and iridium, found alloyed with native platinum or in other natural alloys, such as osmiridium, are all valuable metals. Native platinum is often magnetic, particularly when it contains much iron. Value varies. On account of its freedom from rusting, its resistance to chemicals, and its high melting point, platinum is very useful in chemical manufactures and analysis, for certain parts of electrical appliances, for jewelry, etc.  Native platinum is found mostly in alluvial (placer) deposits; also in peridotite and other olivine rocks, and in serpentine, accompanied by chromite.
Native platinum is found mostly in alluvial (placer) deposits; also in peridotite and other olivine rocks, and in serpentine, accompanied by chromite.
Iridosmine or osmiridium
They are a silvery-white heavy alloy of the valuable metal iridium with platinum, osmium, and other metals; it is found with native platinum and gold. The present prices for the metals extracted from these natural alloys are as follows: iridium, palladium, rhodium, ruthenium. Iridium is used mostly to harden platinum, for fountain-pen points, for standard weights, for ends of surgical needles, etc. Palladium-gold alloys are used in dentistry, jewelry, etc. Palladium-rhodium alloys are used in jewelry.
Arsenic
Symbol, As; color, tin-white, but usually tarnished to dark gray; luster, dull metallic; H=3.5; G = 5.63 to 5.73.  Arsenic is brittle; it powders to metallic gray-white. Native arsenic is not a common material, and is not found by itself in commercial quantities, although it occurs in ores of silver, nickel, and cobalt. From such ores, the arsenic is extracted in the form of the oxide, white arsenic, commonly called arsenic, which sells at 3 cents to 7 cents a pound.
Arsenic is brittle; it powders to metallic gray-white. Native arsenic is not a common material, and is not found by itself in commercial quantities, although it occurs in ores of silver, nickel, and cobalt. From such ores, the arsenic is extracted in the form of the oxide, white arsenic, commonly called arsenic, which sells at 3 cents to 7 cents a pound.
Bismuth
Symbol, Bi; color, silvery-white, with slightly reddish tint; powder, the same; tarnishes to brass-yellow; H =2 to 2.5; G=9.70 to 9.83; cuts easily; cleavage, perfect.
All the minerals so far described are metals (or near-metals), and all have the peculiar shining appearance called metallic luster. Compare the specimens of native metals with those of non-metals. sulphur or diamond for instance. Among the non- metals, graphite is exceptional in having the metallic luster.
List Native NON-METAL Elements
Sulphur
Symbol, S; color, commonly yellow, but sometimes brownish, greenish, reddish, or gray; luster, resinous; H = 1.5 to 2.5; G = 2.05 to 2.09; rather brittle; powder, white; melts easily; burns with a blue flame and choking gas. Sometimes found in crystals of the orthorhombic system. (See Fig. 20).
Native sulphur is found in volcanic districts, in deposits from hot springs, in gypsum beds, and very extensively in limestone in Texas and Louisiana. It is used in the manufacture of sulphuric acid and many other chemicals, and in the manufacture of wood pulp by the sulphite process. Sulphur is now being produced as a by-product from cleaning natural gas in Alberta.
Diamond
Symbol, C; color is ordinarily white, but various other colors occur; H = 10; G = 3.516 to 3.525; luster, adamantine, i.e., very brilliant, but the natural surfaces are often rough and dull. The diamond is a kind of carbon, and is found in crystals belonging to the cubic system, often rounded; cleavage (octahedral) is very perfect. Carbonado is black diamond, amorphous or confused
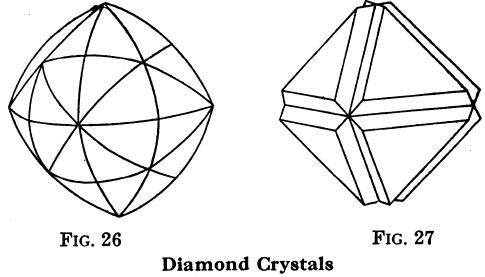
in its crystallization; it is used for diamond drilling etc. Bort is diamond too dull or detective for use as a precious stone; it is used for glass-cutting, diamond-drilling, etc. Diamonds burn when heated strongly in air or oxygen. Very small diamond crystals have been made from charcoal. The value of clear crystals is large, and it increases very rapidly with the size. Diamonds are found in placer diggings, but most abundantly in South Africa in volcanic “necks” or “pipes,” which are filled with decomposed rock derived from peridotite.
Graphite
Symbol, C; color, iron-black or dark steel-gray; H = 1 to 2; G = 2.09 to 2.23. Graphite is also a kind of carbon, but crystallizing in the hexagonal system; other names for graphite are plumbago and black lead. Cleavage, perfect, but not apparent in very fine-grained varieties; luster, metallic, very bright when crystals are large, but dull in fine-grained varieties; but the luster appears when the specimen is rubbed with a knife. Graphite makes a gray mark on paper, and it has a greasy feel. Coke is composed of carbon not crystallized. Considerable quantities of artificial graphite are made from coke by using the electric current to heat it to a very high temperature, so as to cause it to crystallize. Graphite is used for lead pencils, crucibles, lubricants, foundry facings, etc. In the market, two classes of graphite are distinguished: flake, or crystalline, and amorphous. Although not elements, coal, petroleum, and amber may be conveniently described here.
Coal
Coal is composed more or less of the element carbon, but (especially in the softer varieties) mixed with compounds of carbon. First-class anthracite coal is mostly carbon (up to 95%); it is amorphous, i. e., not crystalline. All varieties of coal contain non-combustible substances that are left behind as ash when the coal is burned. Coal has been formed by the slow change, under water at first, of accumulations of plant remains. The first stage is peat, which is still being formed. The peat of past ages has slowly changed into lignite, then into bituminous coal (soft coal), and finally into anthracite, these varieties shading into one another. Ordinary peat shows the plants of which it is composed, matted together. In lignite, the forms of the plants have mostly been destroyed, still more so in ordinary bituminous coal, while in anthracite, the bodies of plants are rarely seen. True coal has not been found in rocks older than those of the Devonian period, and only very little has been found in Devonian rocks.
Anthracite
Anthracite is black, of bright luster; powder is black; breaks with rounded surfaces (conchoidal fracture); composition, from 80% to 95% carbon, the remainder being mostly ash and carbon compounds ; H = 2.5 to 3.5; G = 1.4 to 1.7.
Bituminous coal (soft coal)
Bituminous coal (soft coal) is black; powder is black. It differs from lignite in containing less oxygen and less water, and it differs from anthracite in giving, when heated, more oily and tarry substances, which cause it to burn with a smoky flame. It breaks more easily than anthracite, and often shows a layered structure. H = 2 to 3; G = 1.2 to 1.4.
Lignite (brown coal)
Lignite (brown coal) is from brown to black in color; powder is brown or black; contains from 15% to 36% of water, and shades into ordinary bituminous coal; H = 1 to 2; G = 1.2 to 1.4 Jet is a black variety, capable of taking a high polish. It is used for ornaments.
Peat is mostly made up of decaying bodies of mosses; when dried, it is a good domestic fuel. When pulped and air-dried, it forms a solid mass, which might be used in place of coal to a large extent; but it is not practicable to transport it long distances, on account of its brittleness.
Petroleum
Petroleum is an oil, thin, though sometimes thick (viscous),—usually of a greenish- brown color, but sometimes colorless, yellow, brown, or nearly black; it finally grades into asphalt. Since its specific gravity is from 0.6 to 0.9, it floats on water. Petroleum is a mixture of a great many compounds of carbon and hydrogen. Natural gas, which is found in petroleum fields, is a mixture of similar compounds of carbon and hydrogen; it contains in addition hydrogen and other elementary gases, and sometimes helium.
Amber
Amber is a fossil resin, usually of a yellow color, but sometimes reddish, brownish, or whitish; H = 2. to 2.5; G = 1.096; when heated, it softens and melts. Amber is composed of carbon, hydrogen, and oxygen; it has been found near and on the shore of Cedar Lake, in Northern Manitoba.
