Table of Contents
The US Bureau of Mines is conducting core leaching experiments with sulfuric acid on oxide copper ores from the Cyprus Casa Grande and Asarco-Freeport Santa Cruz deposit in Arizona to further the understanding of the leaching process in supergene copper ores as it relates to in situ mining. Leach solutions from atacamite mineralization contained 5 to 30 g/L (0.05 to 0.28 oil gal) copper and few extraneous ions. Leachant from chrysocolla ores, with grades similar to the atacamite, contained 2 to 9 g/L (0.02 to 0.08 oz/gal) copper and abundant extraneous ions. Quartz and K-feldspar had no impact on leach solution composition at any acid concentration up to 40 g/L – (0.39 oz/gal) while illite and sericite had minimal impact. Biotite, calcium-rich clay, and limonite have a significant impact on solution chemistry when there is abundant free acid in solution. High acid concentrations increase acid consumption in ores with abundant biotite and limonite gangue. High acid concentrations can be used in ores with quartz, K-feldspar, and sericite gangues without increasing acid consumption per unit of copper removed.
The US Bureau of Mines, as part of its in situ mining research, is conducting laboratory leaching experiments on core specimens of selected supergene copper ores. The objective is to document the mineralogical and geochemical changes that occur during in situ mining of oxide copper ores with sulfuric acid. The experimental variables include acid concentration, pressure, temperature, flow rate, ore grade and copper mineralization, gangue mineralization, and textural relationships of ore mineralization, and fluid flow channels. These experiments are not intended to model in situ mining on a field scale using a piece of core. It is assumed that, for example, the reaction of biotite or chrysocolla with sulfuric acid will be the same in the laboratory as it is in the field. The leaching behavior of individual minerals during in situ mining can be established in the laboratory. It is anticipated that what is established in the laboratory will help define a better concept of what can be achieved in the field.
The laboratory research portion of the Bureau’s in situ mining program encompasses two main areas: core leaching, and mineralogical and geochemical studies of pre- and post- leach petrographic thin sections using a JEOL 733 electron microprobe. The leaching experiments use both split and unsplit 5-cm-diam (2-in.-diam) core samples (as opposed to crushed samples). It is believed that data obtained from core leaching experiments will more closely reflect the conditions and reactions occurring during in situ mining.
In core leaching, the flow channels through which leach solutions travel consist of fractures and pore spaces within the rock. In many cases, this is also where ore mineralization is concentrated. Therefore, the proportion of ore minerals contacted by the leach solutions will be greater than in a packed column experiment. This can result in significantly different leach solution composition. Thus, it provides information that may be more directly applied to field predictions.
Whole core experiments also enable permeability changes to be monitored and evaluated with respect to their impact on in situ mining. The mechanisms that influence permeability include ore mineral dissolution, secondary mineral precipitation, clay hydration, and physical sedimentation. When two or more of these mechanisms are operating simultaneously, there may be no net change in permeability. Permeability changes can be either beneficial or harmful to a leaching operation. Net permeability decrease may result in decreased flow capacity, but, improved solution distribution. Likewise, net permeability increases may result in decreased solution distribution, but increased flow capacity.
Methods
General statement
A wide variety of initial acid strengths were used in these experiments. Each experiment is identified by initial acid concentration. Thus, in the 40 g/L (0.37 oz/gal) (atacamite) experiment, 40 g/L (0.37 oz/gal) sulfuric acid was introduced into the sample.
Experimental
An axial flow experimental system was designed to accommodate core specimens (Paulson, et al., 1987). The reaction cell is constructed of acrylic pipe, thereby making it both inert and relatively inexpensive. It was necessary to design the system so that the post-leach samples could be recovered intact for analysis with the electron microprobe. Prior to placement in the reaction cells, the core specimens are cast in an epoxy jacket. The jacket constrains the flow of leach solution within the core. It also helps maintain sample integrity for post-leach tests, as some samples undergo significant degradation during leaching. The leach solution is delivered to the samples by means of a 12-channel peristaltic pump. This pumping system enables a maximum of 12 separate experiments to be conducted simultaneously at varying flow rates. Different flow rates are achieved by varying the tubing diameter for each experiment. Inline pressure gauges on the input and output ends of the samples are used to monitor the differential pressure across the sample. The pressure measurements are then used in permeability calculations. The system can be used at pressures up to 340 KPa (50 psi). An elevated pressure core leaching system has been designed and is currently undergoing testing.
Analytical
Fluid samples are collected at about 48-72 hour intervals and analyzed for SiO2, Al, Fe, Cu, Mg, Ca, Na, K, SO4, and Cl concentrations using atomic absorption spectroscopy, optical emission spectroscopy, and ion chromatography. In addition to pH measurements, a titration is performed on the fluid samples using a buffer technique to determine the free acid content of the fluids. This technique complexes the cations in solution, thus eliminating hydrolysis effects on pH. Therefore, the free acid is a measure of the hydrogen ion that remains in solution after reaction with the rock excluding the contribution due to hydrolysis of cations mobilized during leaching.
Leaching Atacamite Mineralization
General statement
Leach solutions recovered from atacamite mineralization are higher in grade and contain fewer extraneous ions than those recovered from chrysocolla ore. This is in part due to the distribution of copper-bearing minerals in the two ores. A significant fraction of the copper in the Cyprus chrysocolla ore is disseminated. Nearly all the copper in the Santa Cruz atacamite ore is concentrated in fractures. Thus, lixiviant contacts more copper mineralization relative to gangue in the atacamite ores than in the chrysocolla ores.
Atacamite in Asarco – Freeport Deposit
The atacamite (Cu2Cl(OH)3) ore tested was from the Asarco//Freeport Santa Cruz deposit, Pinal County, AZ. This property is an undeveloped porphyry copper deposit buried beneath approximately 305 m (1000 ft) of alluvium and indurated gravel. There are three principal rock units: older Precambrian quartz monzonite (equivalent to Oracle granite),
younger Precambrian diabase, and Laramide biotite quartz diorite porphyry. The supergene ores are characterized by stockwork atacamite mineralization hosted by the quartz monzonite and clay-altered biotite quartz diorite porphyry, stockwork chrysocolla ores hosted by quartz monzonite, and disseminated chrysocolla ores hosted by biotite quartz diorite porphyry. Scattered bodies of chalcocite mineralization are present above and below the main oxide ore zone.
Atacamite mineralization typically consists of a network of mineralized fractures cutting coarse to medium grained quartz monzonite. The modal mineralogy of the quartz monzonite is 40% quartz, 25% K-feldspar, and 20% sericite forming the hypidiomorphic matrix of the rock. The fracture- hosted minerals present are 5% atacamite, 5% limonite (composed of goethite, hematite, and leucoxene), and 5% clay. Illite and halloysite are the principal clay minerals.
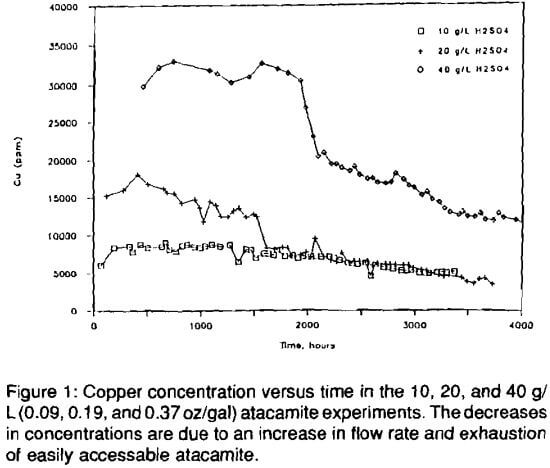
Core specimens of atacamite mineralization were leached with sulfuric acid with initial concentrations of 10, 20 and 40 g/L (0.09, 0.19, and 0.37 oz/gal). Figure 1 illustrates the copper concentration versus time. After 500 hours, copper concentrations were approximately 6, 15, and 30 g/L (0.06, 0.14, and 0.27 oz/gal), for the 10, 20, and 40 g/L (0.09, 0.19,

and 0.37 oz/gal) experiments, respectively, roughly proportional to initial acid concentration. The copper concentration in the 20 g/L (0.19 oz/gal) experiment, at approximately 1600 to 1800 hours, stabilized at the same level as the 10 g/L (0.09 oz/gal) experiment. There was an abrupt drop in copper concentration in the 40 g/L (0.37 oz/gal) experiment, at about 2000 hours. There was no abrupt change in copper concentration in the 10 g/L (0.09 oz/gal) experiment. The gradual decrease in copper concentration observed in all three experiments is attributed to depletion of the easily accessible copper, rather than a change in flow rates.
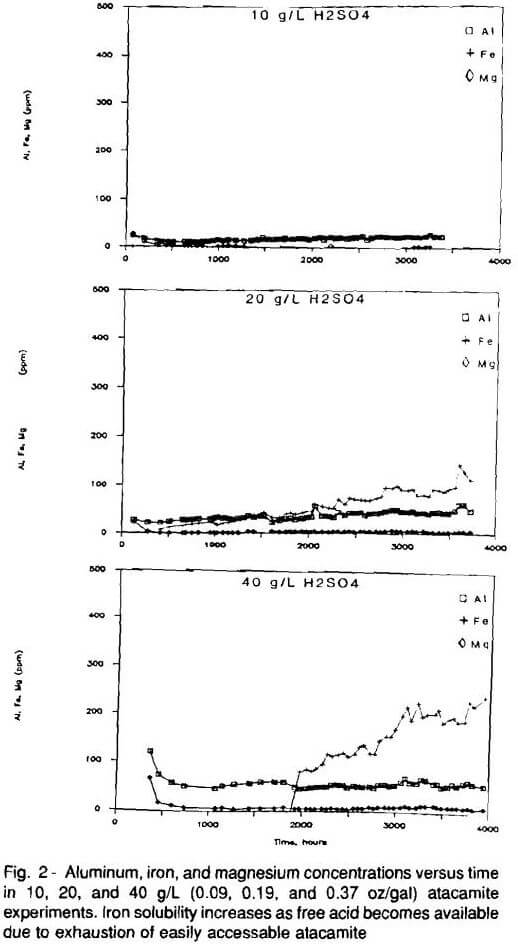
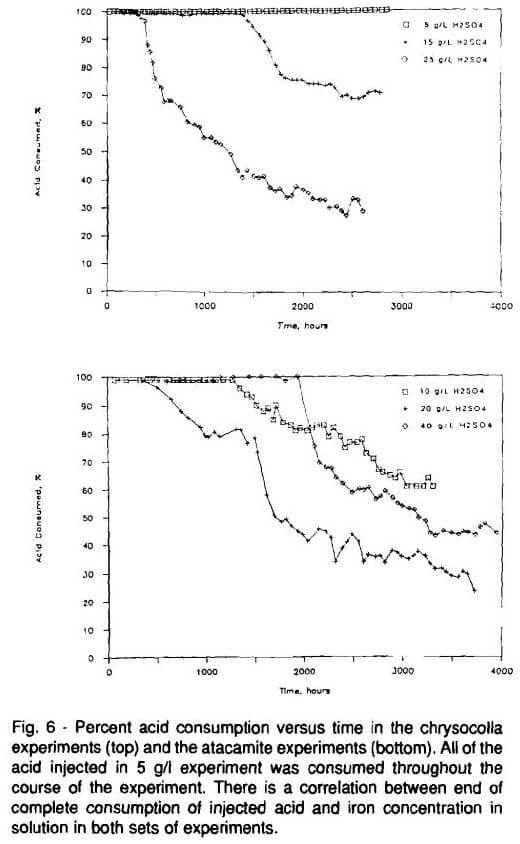
There is a change in iron concentration in the 20 and 40 g/L (0.19 and 0.37 oz/gal) experiments after 2000 hours (Fig. 2). The sudden increase in iron concentration is due to increased dissolution of limonite, the principle iron-bearing mineral in these ores. It is believe an effect similar to buffering, caused by atacamite, inhibits limonite dissolution in the first 2000 hours of the experiments. There is little limonite dissolution as long as most of the available acid is consumed by atacamite dissolution (Fig. 5). However, as atacamite is depleted, resulting in excess free acid, pH drops and limonite dissolution begins. All of the acid available in the 10 g/L (0.09 oz/gal) experiment was consumed throughout the duration of the experiment. Hence, there was little evidence of limonite dissolution during the 10 g/L (0.09 oz/gal) experiment.
It appears that if sufficient free acid is present, limonite dissolution will occur. However, as long as abundant atacamite is present, it consumes most of the available acid and limonite dissolution is inhibited. However, when insufficient atacamite is present to cause the buffer-like effect, limonite dissolution accelerates.
Post-leach study with the electron microprobe revealed no evidence of quartz or K-feldspar dissolution. Comparison of pre- and post-leach microprobe analyses of illite and sericite demonstrated that these minerals released the calcium, sodium, potassium, aluminum, and magnesium observed in the leach solutions. Figures 2 and 3 display the concentrations of selected cations, other than copper, during the atacamite experiments.
Acid consumption independent of acid concentration
The extent of gangue mineral dissolution was minimal in all three experiments (Fig. 4). Acid consumption per pound of copper was relatively constant in all three experiments, despite changes in the rate of limonite dissolution the 20 and 40 g/L (0.19 and 0.37 oz/gal) experiments. The atacamite mineralization experiments demonstrate that the gangue minerals sericite, illite, and limonite have a minimal impact on acid consumption independent of changes in acid concentration, at least up to 40 g/L (0.37 oz/gal).
Leaching Chrysocolla Mineralization
General statement
Initial copper concentrations in the leach solutions from the chrysocolla experiments are one-third to one-half those of the solutions from the atacamite experiments of similar grade. Aluminum, magnesium, calcium, and potassium are two to 10 times more concentrated in the leach solutions from the chrysocolla ores than those of the atacamite ores. Furthermore, there was a significant delay between the beginning of fluid flow and the mobilization of copper. In contrast, copper was mobilized immediately in the atacamite experiments. A second difference between the chrysocolla and atacamite experiments is that acid consumption in the chrysocolla ores was proportional to acid concentration.
Chrysocolla mineralization from Casa Grande
The suite of chrysocolla ores tested was from the 1100 level of the Cyprus Casa Grande mine (formerly the Noranda Lakeshore mine). The deposit is a Laramide age porphyry copper. Most of the chrysocolla mineralization is hosted by granodiorite porphyry. Some chrysocolla mineralization is hosted by biotitized andesitic wall rocks. The supergene ore bodies are within 15 m (49 ft.) of a mid-Tertiary erosion surface. The ore is locally decomposed to grus and in general has very high matrix permeability for a weathered igneous rock. The high matrix permeability of this ore favorably impacts its leaching characteristics because approximately one- third of the copper in the chrysocolla ore zones is disseminated into altered plagioclase and biotite phenocrysts.
The granodiorite porphyry is characterized by 5%-10% phenocrysts of quartz averaging 2-mm-diam – (0.08-in.-diam), 10%-15% biotite phenocrysts 2-3 mm-diam – (0.08-0.12 in. diam), and 25%-40% phenocrysts of plagioclase 2-3 mm- diam – (0.08-0.12 in. diam). The groundmass is principally K-feldspar and quartz with variable amounts of fine-grained biotite. The modal mineralogy of typical oxide ore is 33% quartz, 17% K-feldspar, 30% plagioclase, which is half altered to copper-bearing clay, 11 % biotite, which is half altered to chlorite and clay, 5% limonite (composed of hematite and goethite), and 4% chrysocolla located in fractures.
Typical chrysocolla mineralization is composed of a combination of copper-bearing aluminosilicates. These fall into two categories; chrysocolla and copper-bearing clay. Chrysocolla is principally found in fractures but, is also present in altered plagioclase phenocrysts. Copper-bearing clay is principally found in altered biotite and plagioclase phenocrysts but is present in fractures too. The copper-bearing mineral assemblage in fractures (supergene veinlets) is similar to that in altered plagioclase phenocrysts. The difference between these two occurrences is that chrysocolla dominates the veinlet assemblage while copper-bearing clay dominates in altered phenocrystsChrysocolla has not been identified in altered biotite phenocrysts. The copper-bearing clay minerals found in altered biotite phenocrysts are often magnesium-rich trioctahedral clays as opposed to the aluminum-rich dioctahedral clays found in veinlets and altered plagioclase.
Samples of stockwork and disseminated chrysocolla mineralization from the Cyprus Casa Grande deposit were leached in separate experiments using initial acid concentrations of 5, 10, 15, 20, 25, and 40 g/L (0.05, 0.09, 0.14, 0.19, 0.23, and 0.37 oz/gal). Figure 6 displays the copper concentration verses time for the 5, 15, and 25 g/L (0.05, 0.14, and 0.23 oz/gal) experiments. The 5 and 15 g/L (0.05 and 0.14 oz/gal) experiments were performed on disseminated chrysocolla ore (>50% matrix copper) and the 25 g/l experiment was performed on a stockwork (>50% fracture-hosted copper) chrysocolla ore.
The initial copper concentration from the fracture-hosted ore of the 25 g/L (0.23 oz/gal) experiment rapidly reached its maximum of 9 g/L (1.01 oz/gal) (Fig. 4). There was a very broad peak of relatively high copper concentration, about 5 g/L (0.05 oz/gal), in the 15 g/L (0.23 oz/gal) experiment. The copper concentration in the 5 g/L (0.05 oz/gal) experiment gradually increased until stabilizing at about 2 g/L (0.02 oz/ gal) after 1400 hours. After 2000 hours the copper concentration in all three of these experiments stabilized at about 1.5 to 2.5 g/L (0.01 to 0.02 oz/gal).
The effect of copper distribution (ore texture) is apparent when comparing the 25 g/L (0.23 oz/gal) data with the other two experiments. A 2-3-mm (0.07 – 0.02 in.) thick chrysocolla veinlet ran the length of the 25 g/L (0.23 oz/gal) core. Thus, the ratio of acid to mineralization was very low at the start of leaching in the 25 g/L (0.23 oz/gal) experiment due to the abundance of chrysocolla in this flow channel. The permeability of all three cores was initially the same but, a large increase in permeability of the 25 g/L (0.23 oz/gal) experiment occurred abruptly at 400 hours, the same time that copper mobilization began.
Apparently the increase in permeability observed at 400 hours in the 25 g/L (0.23 oz/gal) experiment was caused by the opening of a channelway due to dissolution of chrysocolla along the veinlet Abrupt changes in permeability were observed in the 5 and 15 g/L (0.03 and 0.14 oz/gal) experiments, but they did not coincide with changes in copper concentration. Thus, depletion of a significant amount of the copper from the veinlet is the probable cause of the precipitous drop in copper concentration, rather than decreased residence time. Copper concentration stabilized at a lower level in the 25 g/l experiment because the leached-out veinlet served as a pipeline for lixiviant through the sample; solution distribution was irregular, therefore less disseminated mineralization was contacted than in the less permeable 5 and 15 g/l experiments.

Figures 7 and 8 display the loadings of cations other than copper in the 5, 15, and 25 g/L (0.03 and 0.14 oz/gal) experiments. Calcium and sodium, which are abundant as exchangeable cations in clays and as impurities in chrysocolla, are concentrated in the first solutions recovered from the chrysocolla ore. The concentration of these two cations in solution is inversely proportional to copper (Figs. 6, 7, and 8). Sodium and calcium are released before copper and apparently are depleted in the rock before copper dissolution begins to dominate the lixiviant rock interaction.
There was little iron in solutions from the 5 g/L (0.03 oz/ gal) experiments (Fig. 7). Comparison of Figs. 6 and 7 reveals that iron concentration began increasing in the 15 and 25 g/L (0.03 and 0.14 oz/gal) experiments when copper concentrations began decreasing. In this respect, the dissolution of iron (from limonite and biotite) in the Cyprus chrysocolla ores was similar to that in the atacamite experiments. There was complete acid consumption throughout the duration of the 5 g/L (0.03 oz/gal) experiment and very little iron in solution. Iron rose in solution in the 15 and 25 g/L (0.03 and 0.14 oz/gal) experiments after easily contacted and dissolved copper was depleted and free acid increased in solution.Electron microprobe analyses demonstrated that, just as in the atacamite experiments, quartz and K-feldspar are unaffected by the low acid concentrations used in these tests. Limonite and biotite were the most reactive minerals in these experiments. Aluminum was released to solution during the dissolution of chrysocolla, copper-bearing clay and biotite. Magnesium, iron, and potassium were released during the reaction of biotite with the lixiviant.
Acid consumption in the chrysocolla experiments
Figure 9 shows the acid consumption in the 5, 15, and 25 g/L (0.03, 0.14 and 0.23 oz/gal) tests. The initial acid consumption was extremely high in all three chrysocolla experiments. However, acid consumption decreased to a minimum of about 3 g acid/g Cu (0.1 oz/0.03oz) in all three experiments. This decrease was most rapid in the 25 g/L (0.23 oz/ gal) experiment due to the added mass of acid available to consume reactive gangue, such as biotite, calcium-rich clay, and limonite.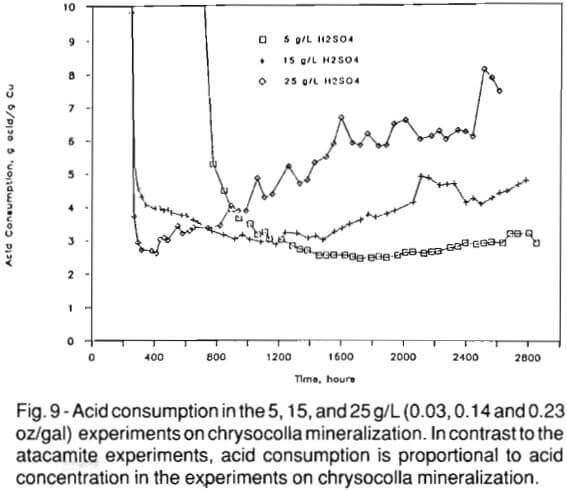
As copper mineralization is depleted, acid consumption per unit of copper dissolved increases (Fig. 9). The rate of increase is proportional to initial acid concentration, being greatest in the 25 g/L (0.23 oz/gal) experiment. Concentrations of extraneous cations in solution indicate that the attack on gangue is more vigorous with increasing free acid concentration. The loadings of aluminum, magnesium, and potassium, all constituents of biotite, in the 5 g/L (0.03 oz/gal) experiment are at levels similar to the atacamite experiments that contained no biotite. These constituents of biotite (Al, Mg, Fe, K) are relatively abundant in the 15 and 25 g/L (0.14 and 0.23 oz/gal) solutions and acid consumption is proportional to aluminum, magnesium, and potassium concentrations. Evidently, the reaction of biotite with the lixiviant in these tests is significant as the key to acid consumption by gangue minerals.
There is evidence that the relatively low acid consumption observed in the lower acid concentration experiments – <15 g/L (0.14 oz/gal) is due to secondary mineral precipitation. Figure 10 is a photomicrograph of typical chrysocolla mineralization after leaching with 10 g/L (0.09 oz/gal) sulfuric acid for more than 3000 hours, which shows an altered phenocryst of biotite at the edge of a leached-out supergene veinlet. A dark layer is visible covering the walls of the veinlet. The layer is continuous and thick along the biotite phenocryst. There are gaps in the layer elsewhere along the veinlet. It is suspected this layer is a secondary precipitate composed of iron, copper, and potassium sulfates. The exact mineralogical and geochemical character of the layer is presently under investigation.
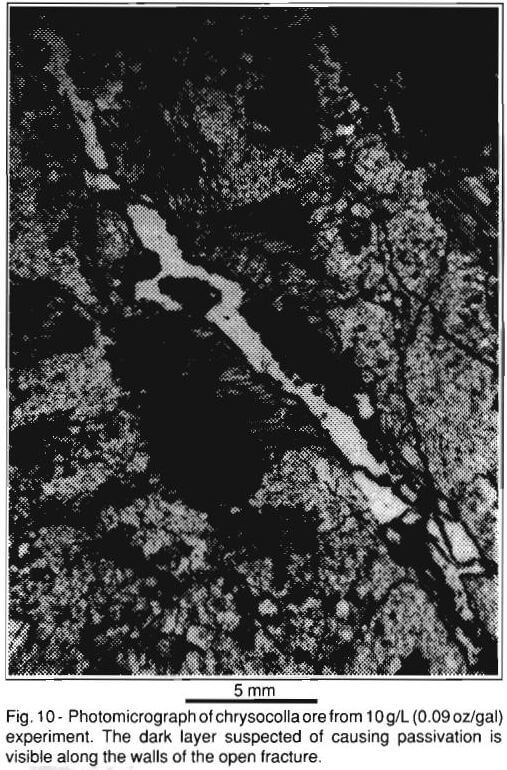
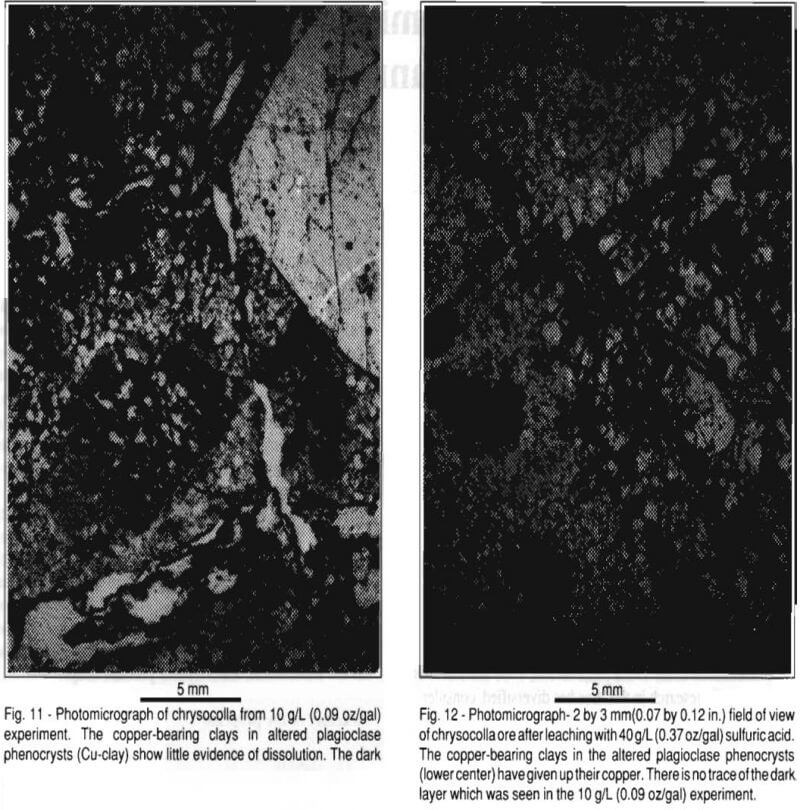
Figure 11 is a photomicrograph of a different area in the same specimen shown in Fig. 10. The dark layer is clearly visible lining the walls of flow channels. Furthermore, open flow channels can be seen passing through a plagioclase phenocryst containing chrysocolla and copper-bearing clay, but there is little evidence of leaching in this phenocryst. It is believed the dark layer may be isolating (passivating) the copper mineralization within the altered phenocrysts. Figure 12 is a photomicrograph of typical chrysocolla mineralization after leaching with 40 g/l sulfuric acid. A leached-out veinlet is shown where it passes through an altered plagioclase phenocryst. The copper mineralization is gone from this phenocryst. Furthermore, there is very little evidence of a dark, passivating layer of secondary precipitates.
The dark layer visible in Figs. 10 and 11 is absent in natural, unleached specimens and in specimens leached at higher acid concentrations – >15 g/L (0.14 oz/gal). It is possible that this layer isolated reactive gangue minerals, such as biotite and copper mineralization from lixiviant in the lower acid concentration – <15 g/L (0.14 oz/gal) experiments. Hence, these experiments had lower acid consumption, but also long delays in copper dissolution and relatively low copper loadings.
Discussion and conclusions
The gangue minerals sericite, illite, and limonite have a minimal impact of acid consumption independent of acid concentration, up to 40 g/L (0.37 oz/gal). Biotite can have a significant impact on acid consumption. Increased copper recovery and recovery rates resulting from higher lixiviant concentrations will have to be weighed against a concomitant
increase in acid consumption in some ore types. Acid consumption in biotite-rich ores is proportional to acid strength. Therefore, it is possible to use too much acid when leaching an ore containing reactive gangue. However, in ores containing unreactive gangue minerals (quartz, sericite, K-feldspar) high acid concentrations can be used to increase copper concentration without a significant impact on acid consumption per pound of copper.
There appear to be two stages of leaching in the Cyprus chrysocolla ores: dissolution from fracture-hosted copper mineralization and leaching of disseminated copper. The early period of fracture-hosted copper leaching produced the highest concentrations of copper observed in the chrysocolla experiments. Leaching of disseminated copper mineralization resulted in low, relatively stable copper concentrations. The matrix permeabilities of the chrysocolla samples are similar, approximately 0.2 millidarcies. Copper loading is not proportional to initial acid concentration when leaching disseminated copper mineralization. Matrix permeability apparently controls the rate at which lixiviant contacts disseminated mineralization and copper is solubilized.
There was a delay of 200 to 600 hours between the beginning of flow and the mobilization of copper in the chrysocolla experiments. In contrast, copper was mobilized immediately in the atacamite experiments.The Bureau identified two possible mechanisms that may be responsible for the observed delay in copper recovery.
First, high calcium in the solutions correlates with the period of no net copper recovery. Microprobe and chemical studies have demonstrated that the clay minerals in the chrysocolla ore have a predominance of calcium in their exchangeable sites. Therefore, ion exchange would result in acid consumption and the liberation of calcium. Thus, the consumption of acid by extraneous cations more soluble than copper, such as calcium from clays, left insufficient acid for the mobilization of copper.
Second, high calcium concentrations and elevated pH resulted in the precipitation of gypsum from the initial fluid samples. Microprobe studies reveal the presence of gypsum and chalcanthite in biotite after reaction with lixiviant. Thus, it is possible that copper mineralization was dissolved from the outset of the experiments but, copper mobility was inhibited by secondary precipitation until pH decreased to a level that allowed copper transport. Although precipitation was taking place there was a gradual increase in permeability in all the experiments.
In extrapolating this information to an in situ mining operation Bureau of Mines researchers believe there are two salient points. There is an optimum initial acid concentration for the in situ mining of ores that contain abundant biotite, calcium-rich clays, and limonite. Also, mechanisms that inhibit copper mobility will operate at the edge of a leach field and during restoration because of ground water dilution of lixiviant.
