Acid mine drainage sludge is produced when an acid drainage stream is treated with lime, sodium hydroxide, ammonium or sodium carbonate in order to neutralize the acidity of the solution. It was found that a sludge sample taken from southern West Virginia contained significant amounts of nickel, cobalt and manganese. Nickel was 0.26%; cobalt 0.21%; and manganese, 4.5%. Such high values of nickel and cobalt have not been previously reported in acid mine drainage sludges.
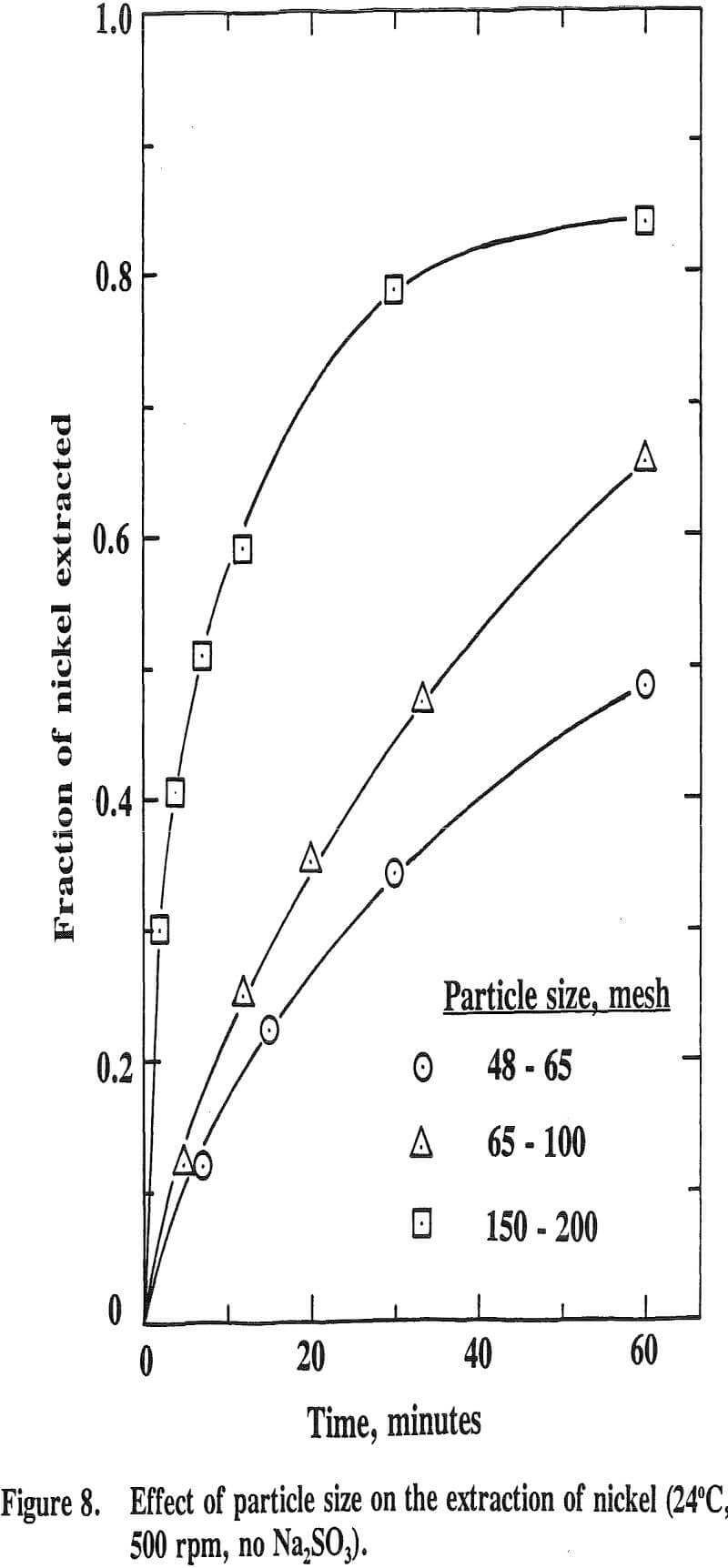
The sludge was the one which had been produced by adding sodium hydroxide to the acid mine drainage. The sample was filtered, dried, crushed, and sized into 48-65, 65-100, 100-150, 150-200, and minus 200 mesh fractions. Each size was analyzed for various metals. The average contents of the metals were 4.4% Mn, 0.26% Ni, 0.21 % Co, 0.7% Zn, 1.83% Fe, 8.3% Mg, 0.41 % Ca, and 0.17% Cu. The acid insoluble content was 15.3%.
Experiments were conducted in a one-liter cylindrical reactor contained in a thermostated oil bath. The concentration of the sulfuric acid solution was 1 or 2 normal. The volume of this solution was recorded and analyzed for acid consumption.
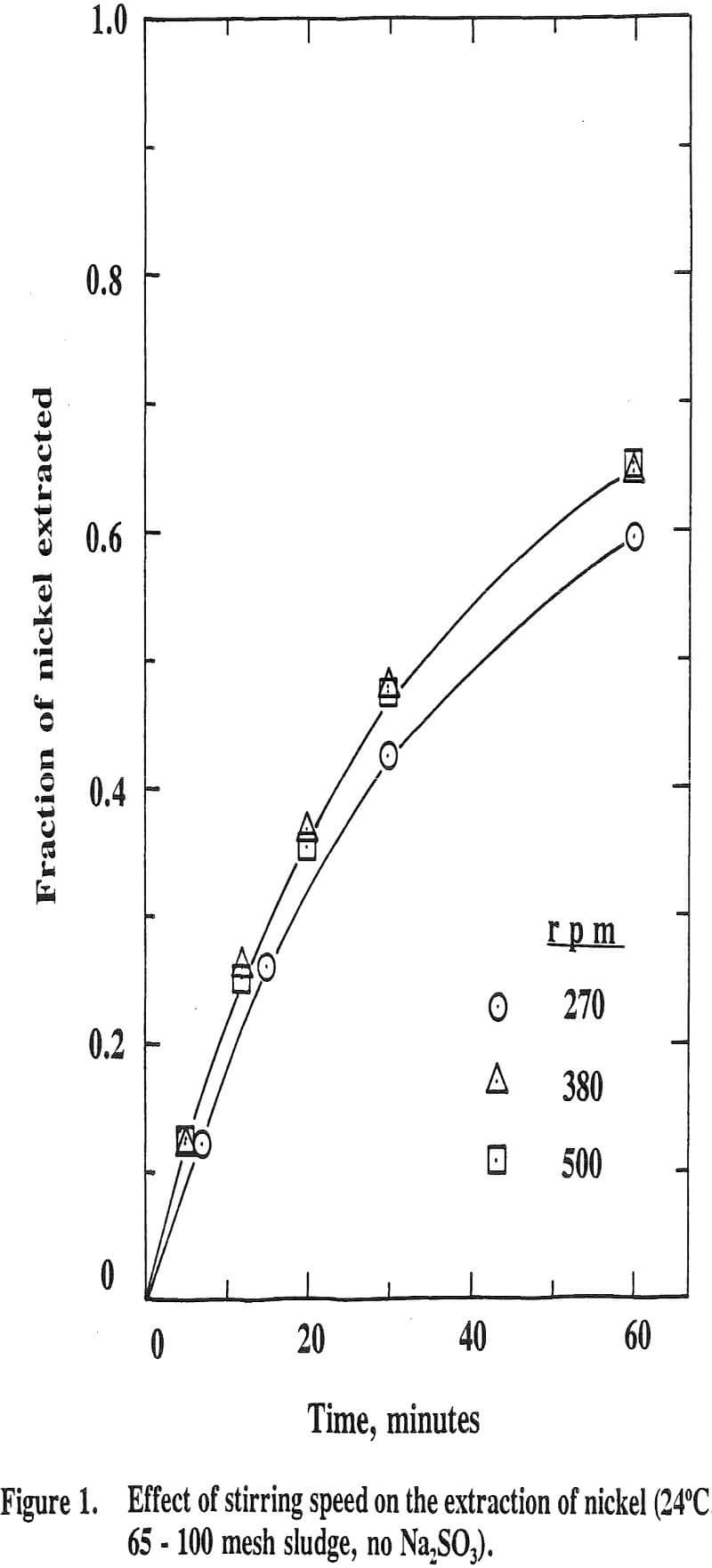
The stirring speed was varied from 270 to 500 rpm. The extraction increases with increasing the stirring speed from 270 to 380 rpm; however, it levels off upon further increasing the speed to 500 rpm. Thus, all the subsequent experiments were carried out at this rpm with a view to eliminating film diffusion controlling mechanism.
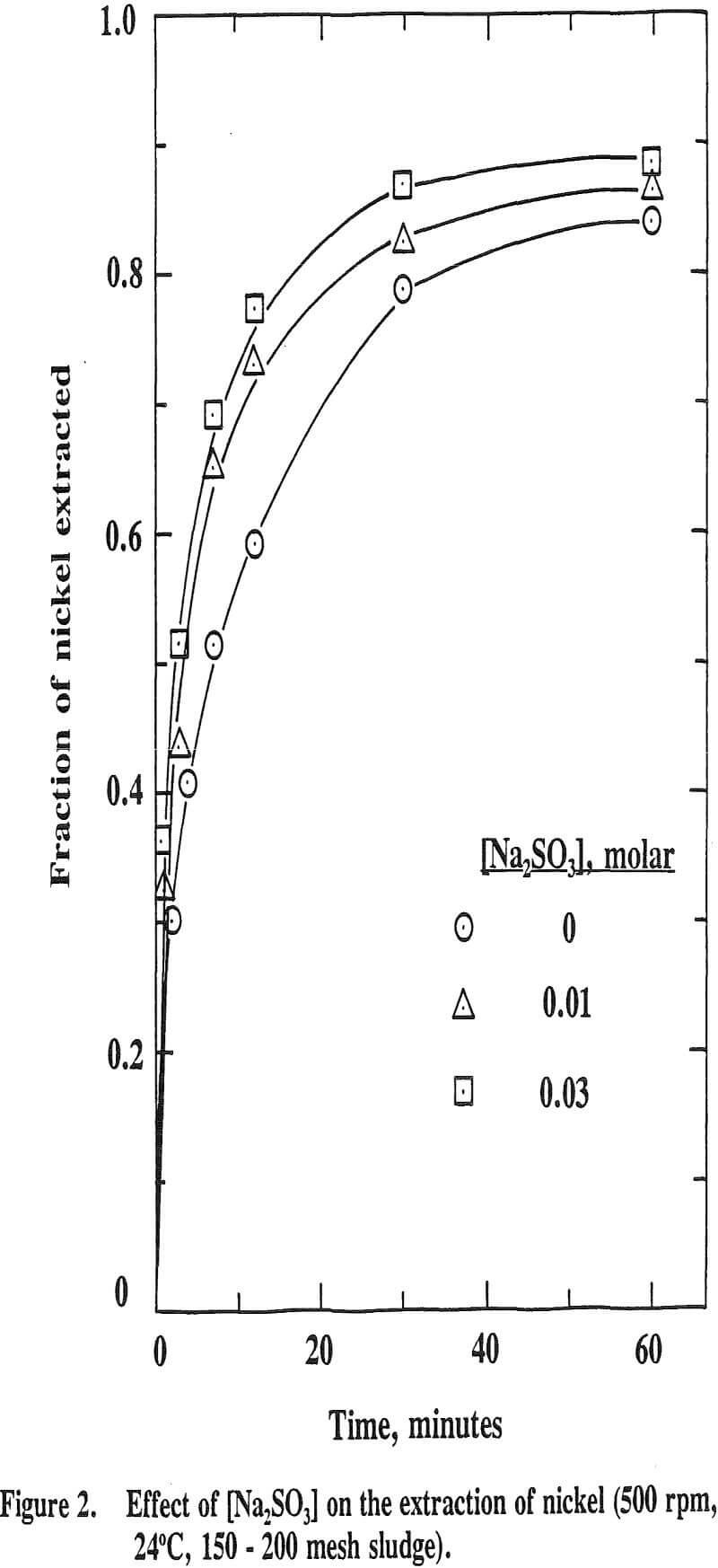
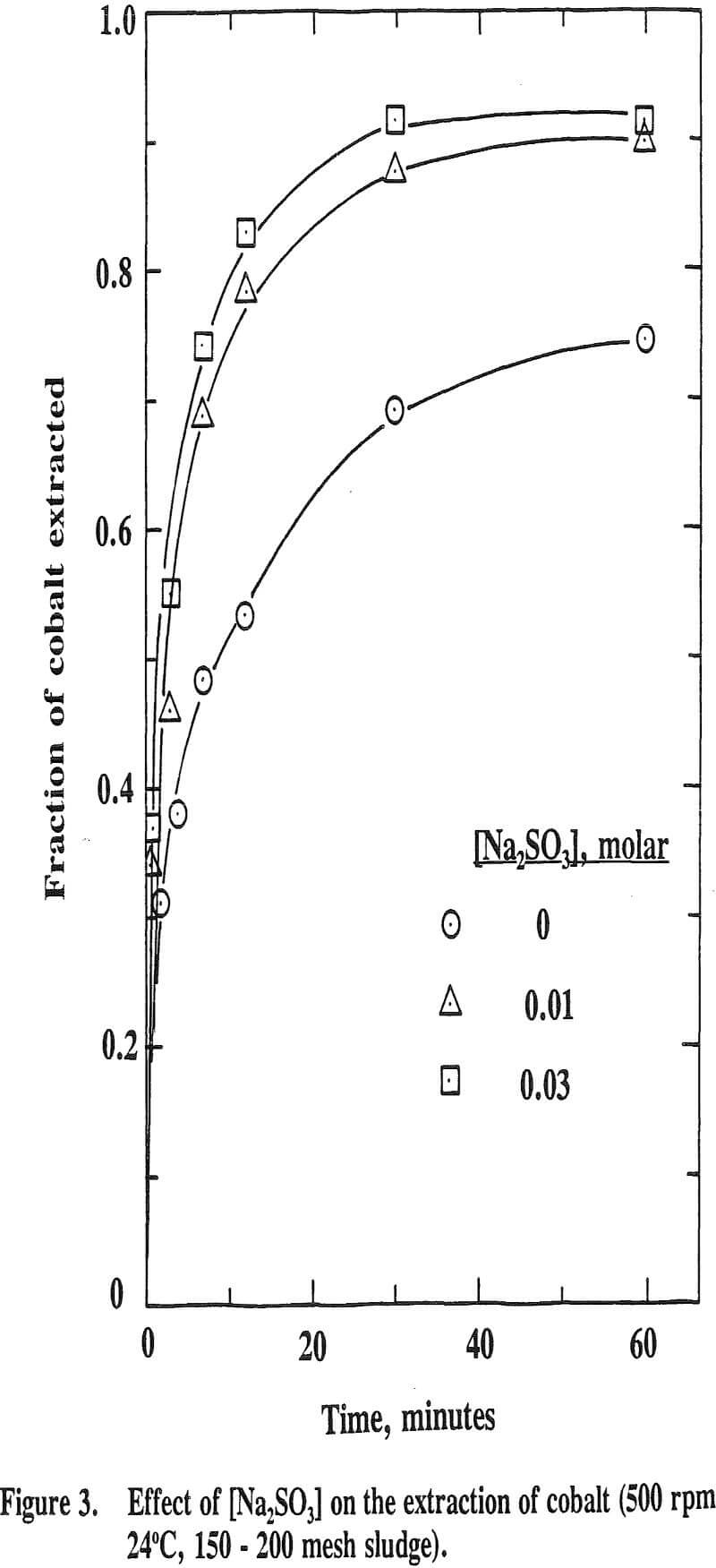
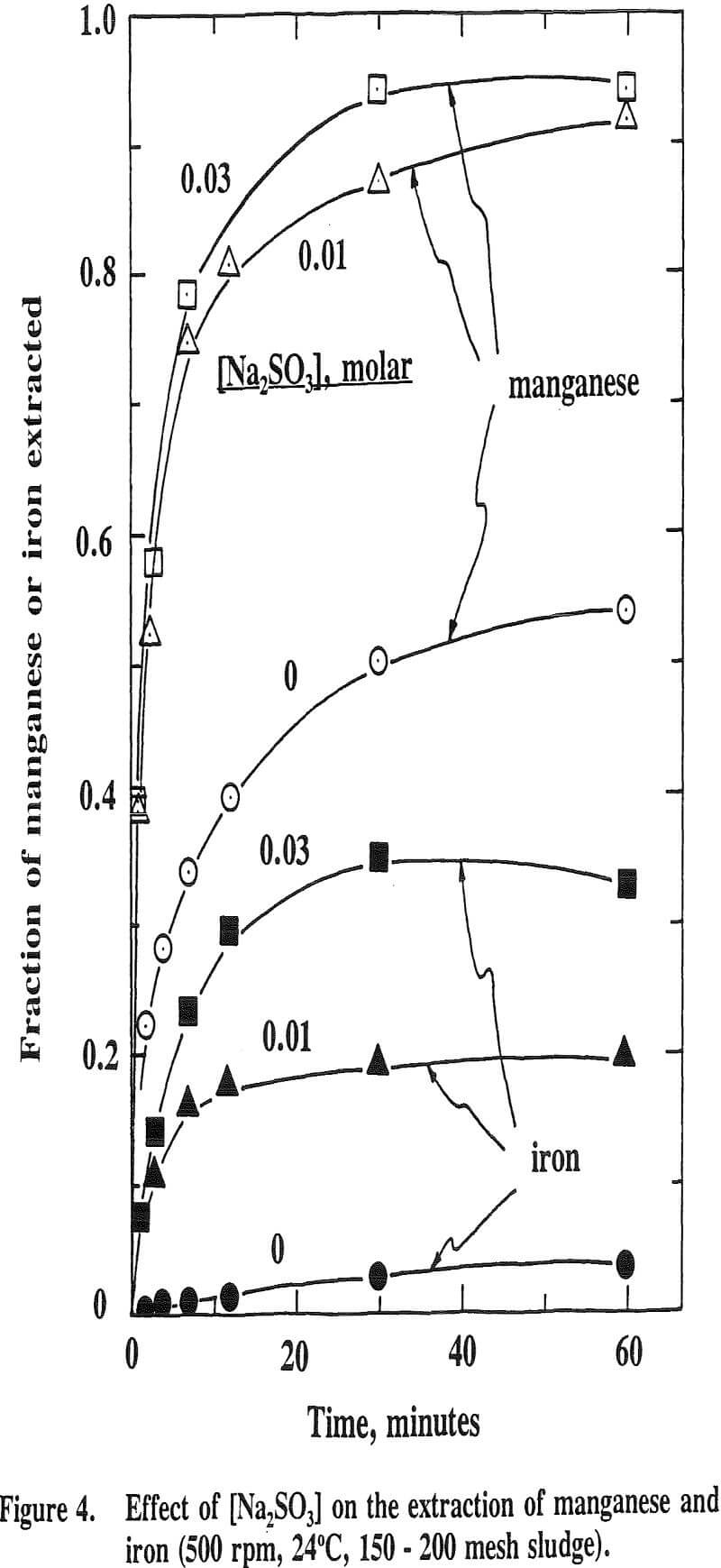
It is well known that Ni, Co and Mn exist in the natural state at divalency as well as at higher valencies. Eh-pH stability diagrams show that Ni3O4, Co2O3 and MnO2 are the probable species existed at higher valencies. Thus, a reducing reagent is needed to leach these oxides. Sodium sulfite was added as a reducing reagent in this study.
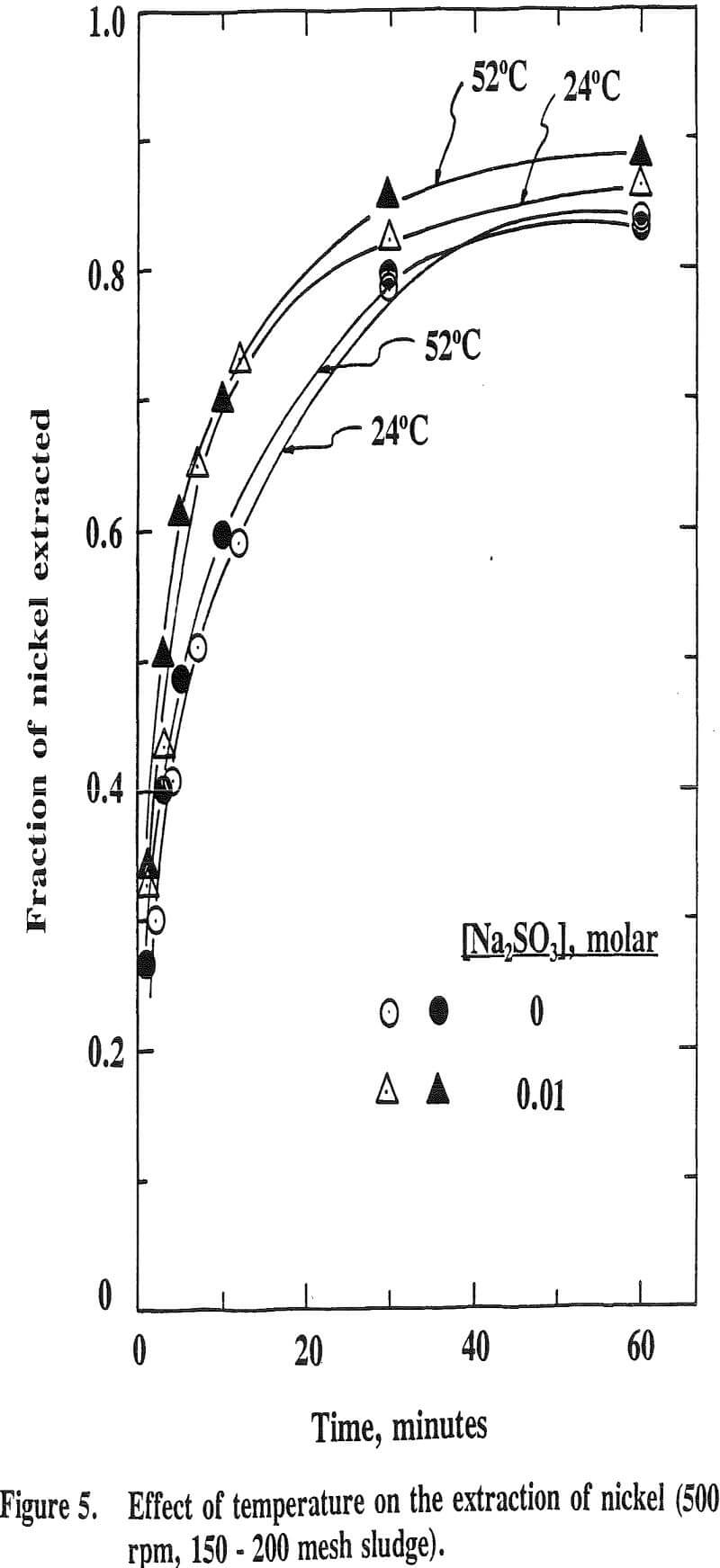
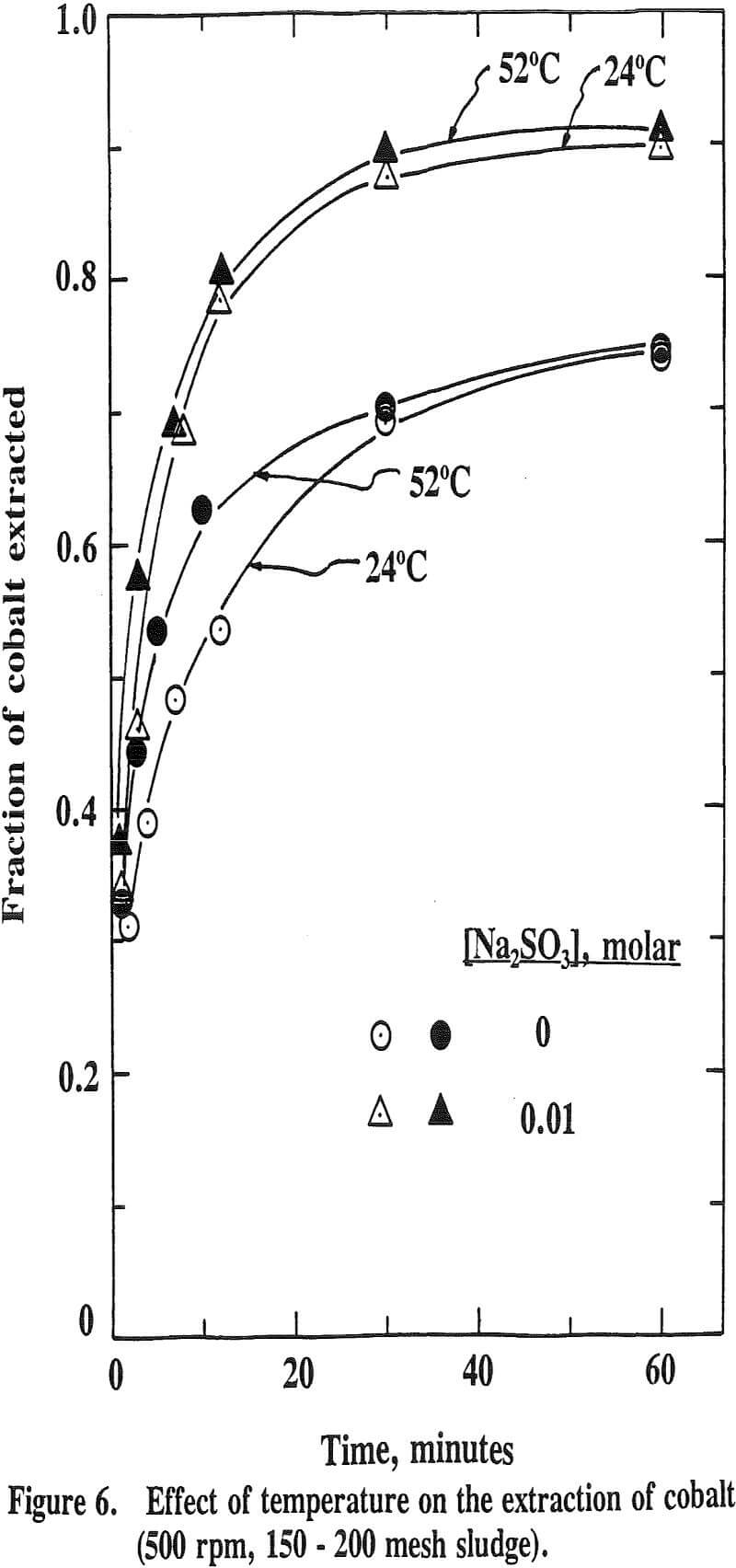
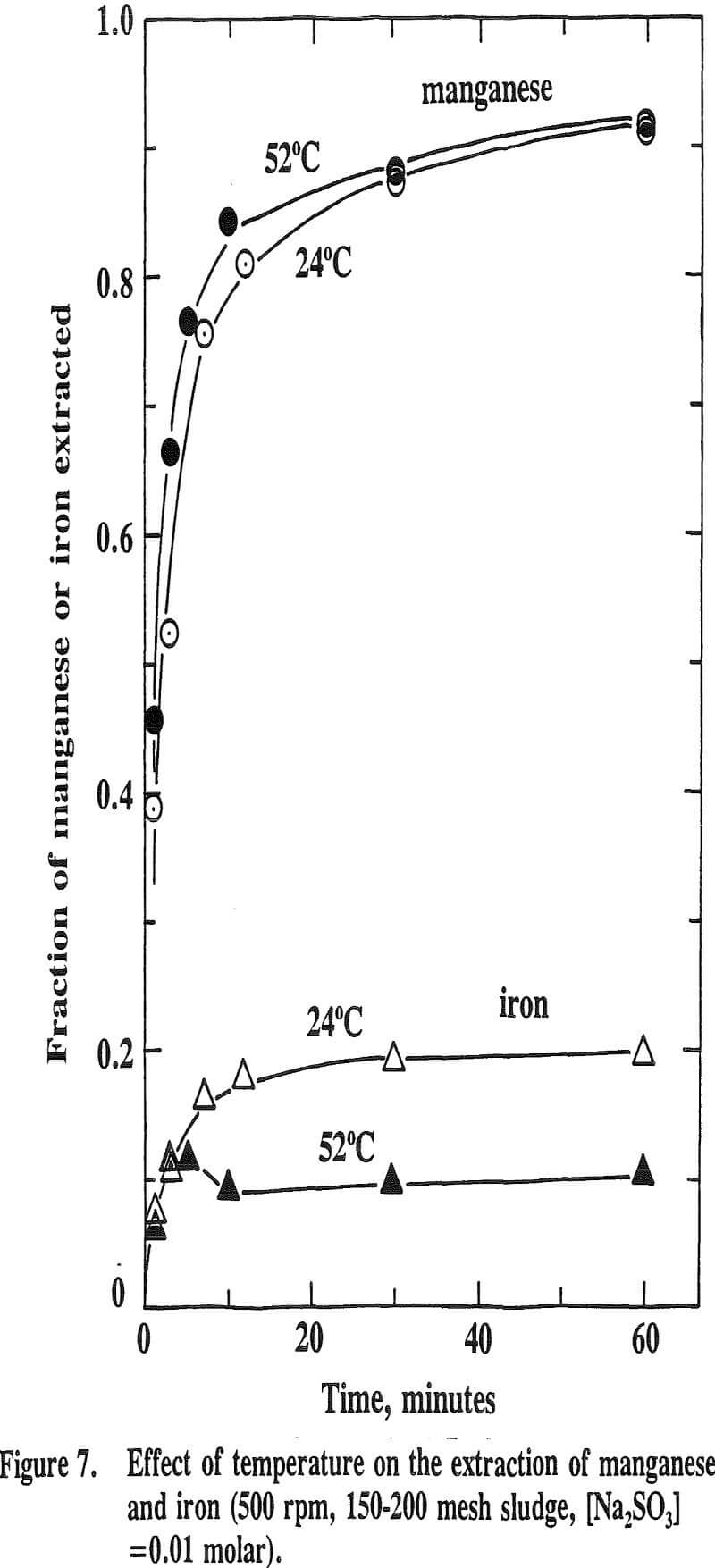
The effect of temperature on the extraction of iron is such that the extraction decreases with increasing temperature. This is the opposite effect for nickel, cobalt and manganese. This is due to the fact that the oxide phases, iron oxide in particular, become increasingly stable as the temperature increases. It has been reported that the stability of oxide phases follows the order Fe>Mn>Co>Cu.
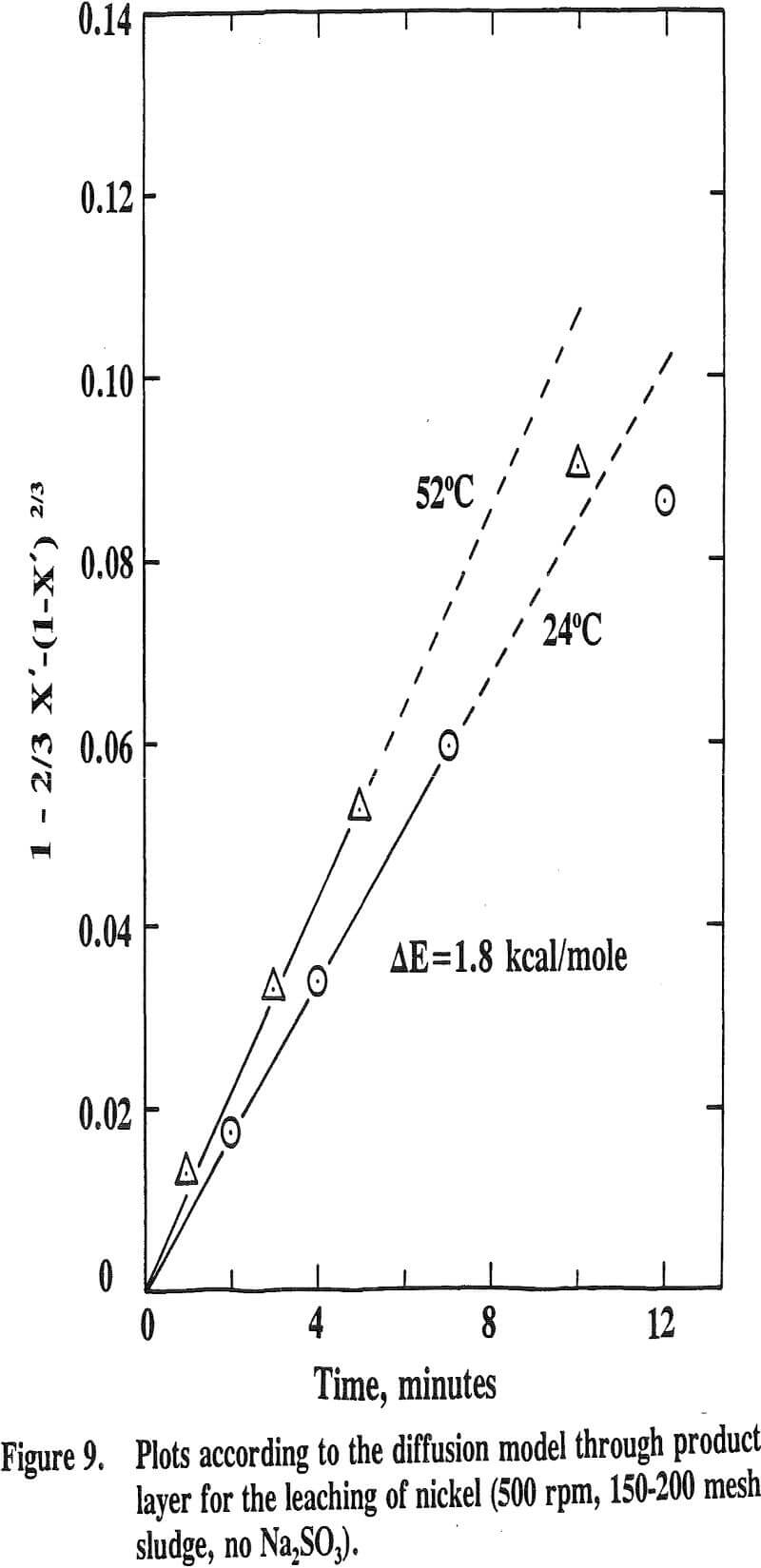
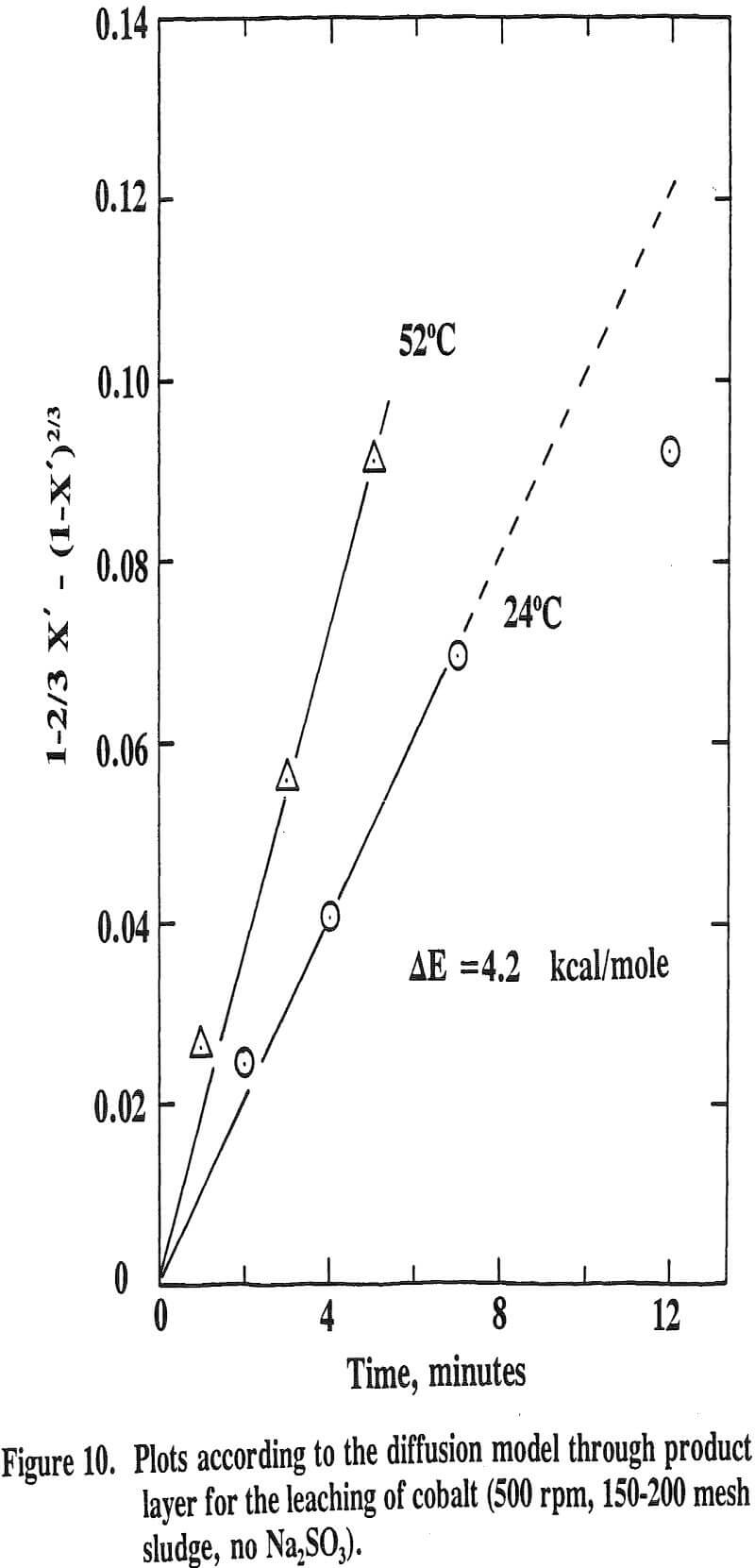
It has been reported that the leaching of nickel with sulfuric acid from a manganese nodule is very temperature sensitive with an activation energy higher than 10 kcal/mole while that of cobalt is only slightly influenced by temperature with an activation energy lower than 5 kcal/mole.