The Bureau of Mines conducted column-leaching tests on 25 domestic Mn ores to determine the feasibility of extracting Mn by in situ or heap leaching with dissolved sulfur dioxide (SO2) solutions. The ores tested were obtained from deposits in Arizona, Arkansas, California, Colorado, Maine, Minnesota, Nevada, and South Dakota.
Dissolved SO2 was found to be effective for leaching Mn; extractions greater than 90 pct were obtained with solution application rates of 1 mL/min from uncalcined, minus 2.5- plus 1.3-cm ore pieces. Leaching these ores with solutions containing only 5-wt-pct SO2 and no added H2SO4 resulted in Mn being selectively extracted over Fe except when Fe was present as goethite or siderite. Ca solubilization was inhibited by controlling the solution application rate (1 mL/min). The rate of leaching was found to be principally dependent upon the rate at which the leaching solution can penetrate the ore, since the solubilization of Mn upon contact with SO2 was observed to be rapid. Despite low water permeabilities of the ores (as low as 10-² to 10 -4 darcy), greater than 90 pct of the Mn was extracted from even the largest ore pieces tested (approximately 10 cm in diameter). It appears, therefore, that leaching Mn opens up the pores, allowing penetration of the leach solution into the ore pieces.
In support of its goal to maintain an adequate supply of minerals to meet national economic and strategic needs, the Bureau of Mines has investigated the feasibility of in situ and heap leaching of low- and medium-grade domestic Mn ores. Mn is a strategic and critical metal that is essential to the steel industry because no other metal has been found to be a satisfactory substitute. The United States has no high-grade deposits of Mn and relies on foreign sources of supply for 98 pct of the Mn it consumes annually. Although plentiful supplies of Mn are readily available from several foreign countries, either as high-grade ore or as ferromanganese, the United States is vulnerable to sudden cessation of foreign shipments during international crises. This, coupled with a widening trade imbalance, has kindled a renewed search for economic methods of producing Mn from domestic deposits. Kilgore and Thomas investigated costs of producing Mn from eight major domestic deposits using various conventional mining and milling schemes. They found that Mn could not be produced by conventional methods at costs that were close to those for Mn ore purchased from foreign sources. The costs (1981) were $8 to $35 per long ton of Mn ($0.16 to $0.17 per kg) for domestic deposits, while those for foreign sources averaged between $1.60 and $1.70 per long ton unit of contained Mn ($0.03 per kg) for 48 pct minimum-grade imported Mn ore f.o.b. at Chicago or Pittsburgh.
In situ and heap leaching of domestic Mn ores with aqueous SO2 appears to have present merit for producing Mn for non-steelmaking applications and future merit for all applications in times of emergency in the event that cessation of foreign shipments of Mn is eminent. Heap leaching remains an important method for recovery of Au, Ag, and Cu. In situ leaching technology has been used in the uranium industry with great success and in the copper industry with a lesser degree of success. These leaching technologies have the advantages over conventional mining of lower capital costs, lower operating costs, short startup times, as well as environmental and safety improvements. Thus, heap or in situ leaching is a favorable method for small and/or low-grade deposits that would not otherwise be mined. Most of the more than 2,100 low-grade Mn deposits in 35 States would fall into this category. Mn is present as oxide-hydroxide, carbonate, and silicate mineralization in these deposits.
Previous Research
The literature contains many laboratory and pilot plant investigations of leaching Mn ores and minerals with dissolved SO2. Davis conducted leaching experiments with SO2 solutions on Mn minerals. He examined the leaching responses of minus 0.149- plus 0.070-mm and minus 3.36- plus 1.68-mm samples of pyrolusite (MnO2), psilomelane (MnO2·H2O), hausmannite (Mn3O4), braunite (3Mn2O3·MnSiO3), manganite (Mn2O3), rhodochrosite (MnCO3), and rhodonite (MnSiO3) to a 2-wt-pct-SO2 solution. Mn was completely leached from minus 0.149- plus 0.070-mm samples of pyrolusite, psilomelane, and braunite in 15 min, and from minus 0.149- plus 0.070-mm samples of hausmannite and manganite in 2 and 48 h, respectively. Mn extraction of 94 pct was observed in 48 h for minus 0.149- plus 0.070-mm rhodochrosite. No perceivable extraction was observed from minus 0.149- plus 0.070-mm rhodonite in 48 h. Times for complete extraction of Mn from minus 3.36- plus 1.68-mm samples were 12 h for psilomelane and 24 h for pyrolusite, hausmannite, and braunite. Manganite, rhodochrosite, and rhodonite were 92.5, 97.5, and 0 pct leached in 144 h.
Henn (4) reviewed proposed processes for the recovery of Mn from domestic resources using SO2, H2SO4, or a combination of both for leaching. All these processes involved ground ore ranging from minus 0.841 mm to minus 0.070 mm. Reviewed processes included the following:
- An SO2 drum leach (Cleaver), in which hot SO2 gas contacts moist ore (minus 0.841 mm) in a two-stage drum leach.
- A SO2 tower leach, in which ore pulp (80 pct minus 0.070-mm ore) cascades down packed towers against rising gas containing SO2.
- A jet-leaching method, in which a slurry of ground ore (minus 0.210 mm) is pumped through a jet nozzle into a column with sufficient kinetic energy to draw S02 gas into the slurry stream.
- An SO2 leach with autoclave purification, in which leaching of a minus 0.177-mm ore slurry is done countercurrently to bottom-injected SO2 gas in a baffled, vertical, cylindrical vessel, and the pregnant leach solution is autoclave-oxidized to eliminate dithionates and sulfities.
- A dithionate leach in which a pulp of ground ore (minus 0.210 mm) in a calcium dithionate (CaS2O3) solution is leached countercurrently in an open gas- absorbing-leach cell using a standard turbo-gas-adsorber mechanism in a baffled tank.
- An SO2 leach under catalytic oxidixing conditions (Ryerson modification), in which SO2 is catalytically oxidized to H2SO4 to leach carbonate ore (minus 0.210 mm) countercurrently in a wooden drum or batchwise in a spray leacher.
- An SO2-H2SO4 leach in which pulped ore (minus 0.210 mm) is leached by addition of H2SO4 and gas containing SO2 and air at 65° C and pH 1 to 3.
- An SO2-CaCl2 (calcium cloride) leach in which SO2 is passed through a pulp of crushed ore in a CaCl2 solution to form soluble manganese chloride (MnCl2) and insoluble calcium sulfite (CaSO3).
- An SO2-Fe-oxidizing leach in which ground ore containing Mn in either the tetravalent or divalent state or both is leached with a saturated solution of SO2.
Wyman and Ravitz investigated leaching of various western Mn ores, including ores from Arkansas and Minnesota, using solutions containing 3 to 6 wt pct SO2. Tests of 10 h or less duration were conducted either countercurrently with SO2-air mixtures bubbled through ore slurries, or batchwise with either a SO2- H2SO4 combination leach solution or with dissolved SO2 solutions alone. Most tests were conducted on either minus 0.841 or minus 0.210-mm ore.
Depending upon the Mn mineralization in the ore, extractions of 42 to 99 pct were obtained. The lower extractions were for ores containing braunite and maganite. A 504-h percolation leach on minus 2.5-cm Boulder City (Clark County, NV) ore with an SO2 solution alone resulted in 93 pct extraction. A 43-h percolation leach on minus 1.3-cm Three Kids, (Clark County, NV) ore with a SO2-H2SO4 leach solution resulted in 95 pct extraction. The authors concluded that psilomelane, pyrolusite, and wad (MnO2, MnO, and other hydroxides) were most responsive to SO2 leaching, with braunite, hausmannite, manganite, rhodochrosite, and rhodonite being less responsive, in that order.
Bender and Rampacek conducted percolation leach tests on four wad ores and a typical hard ore from deposits in Arizona and Nevada. Three of the wad ores were prebaked at 600° C to prevent sliming of clays and plugging of columns. The leaching method involved alternating upward passage of SO2 gas through an agglomerated charge of moist ore and downward passage of wash solutions to remove soluble Mn. Extractions of 82 to 97 pct were obtained for ore sizes of minus 2.5 cm, minus 1.3 cm, and minus 0.64 cm in 5 to 7 days.
Larger, tile-column-leach tests made with 100 kg samples of two wad ores and the hard ore resulted in 80- to 96-pct extractions in 1 to 5 days. A heap-leaching study using 364-kg samples (56- by 117- by 76-cm-deep heap dimensions) resulted in an overall extraction of 83 pct from a roasted and agglomerated wad-type ore in 20 h; however, 96.5 pct of the Mn was extracted from 77 pct of the total ore charge by weight. In some sections of the column (23 pct of the total ore charge by weight), only 37.8 pct of the Mn was extracted.
Current Research
Although many laboratory and several pilot plant tests have been conducted on leaching of Mn with SO2 solutions, the present study was different from the earlier studies in several key respects. Evaluation of heap- and/or in situ- leaching systems dictates that larger size ore pieces (greater than or equal to minus 2.5 cm plus 1.3 cm) should be tested. Use of these leaching systems also dictates percolation through ore columns as the preferred testing method rather than batchwise immersion in beakers. Tests conducted on sea nodules showed that lower concentrations of SO2 in leach solutions (<6 wt pct SO2) resulted in more selectivity in the metals leached. A 5-wt-pct-SO2 solution would preferentially leach most of the Mn while leaving in the residue most of the Fe and P that were coleached in other SO2 leaching studies. Finally, the ore was not calcined to improve permeability or to decompose carbonates, nor was H2SO4 added to the leach solution.
In situ leaching of Mn ores with SO2 was not seriously considered until Mr. James Lake (President, Mineral Investments Corporation, Tucson, AZ) presented several ideas on leaching Artillery Peak deposits to the Bureau. Subsequently, a contract was awarded to a private firm to evaluate the technological feasibility of in situ leaching of various metals other than copper and uranium. The favorable conclusion reached by this contract, that in situ leaching of massive manganese-oxide deposits offered potential for commercialization for specialized Mn markets, triggered an in-depth evaluation by the Bureau of the leaching potential of various domestic deposits. The present investigation is a part of that investigation. The basic approach of the Bureau’s evaluation was to—
- Rank deposits based on geologic, hydrologic, and geographic factors.
- Collect samples from the highly ranked deposits.
- Conduct laboratory leaching tests on the samples with aqueous SO2.
- Analyze technical feasibility and economics of leaching several typical types of deposits.
- Conduct field tests to verify laboratory results and certain assumptions in the economic analysis.
The objectives of this investigation were to collect samples from highly ranked deposits and to conduct laboratory leaching tests on the collected samples to determine the ammenability of these samples to SO2 leaching. Batch-leaching tests were conducted on the finer sized ore particles to estimate maximum extractable Mn. Column-leaching tests were employed to simulate in situ- and/or heap- leaching conditions. Recovery of Mn from solution in form used in the chemical, battery, and superalloy industries was also investigated but is not covered in this report. An earlier report described the ranking of deposits and the analysis of the technical feasibility and economics of leaching several typical types of deposits. Budgetary constraints have prevented any field testing to date.
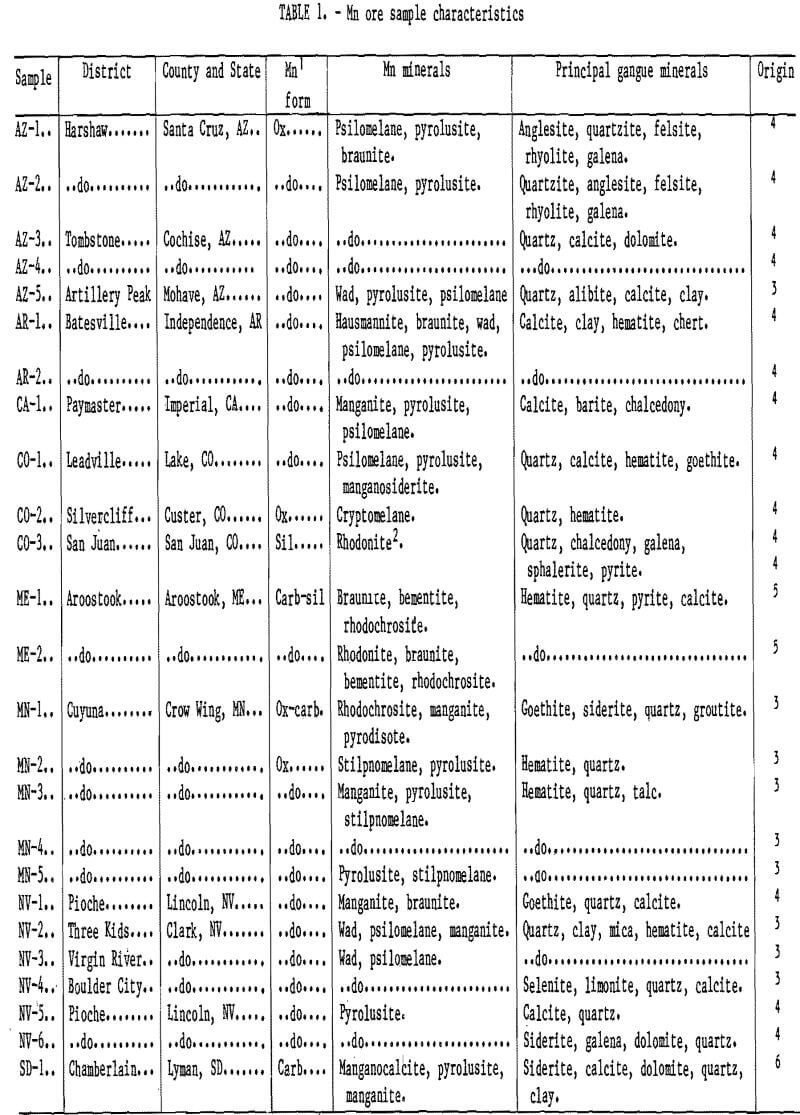
Characteristics of Selected Deposits
The deposits that were sampled and tested in column-leaching experiments are characterized in table 1. The deposits are either of sedimentary or hydrothermal-vein origin and contain predominantly the higher order oxide and hydroxide minerals: pyrolusite, psilomelane, wad, and manganite. The sedimentary deposits are generally massive, low- grade, high-volume deposits, compared to the higher grade and smaller volume vein deposits. With the exception of the Cuyuna deposits, the sedimentary beds are horizontal, while most of the vein deposits are steeply dipped. Partial chemical analyses are given in table 2.
The samples were obtained mostly from road cuts, loose rock, waste rock piles, and lean ore dumps at or around abondoned and, in some cases, active mines. Sample AZ-5 was obtained from an 18.3-m-high exposed section of Cob Web Hill in the Artillery Mountains of Arizona. Sample SD-1 was comprised of nodules handpicked from a stockpile of shale matrix on a dormant Bureau experimental mine site near Chamberlain.
Experimental Methods
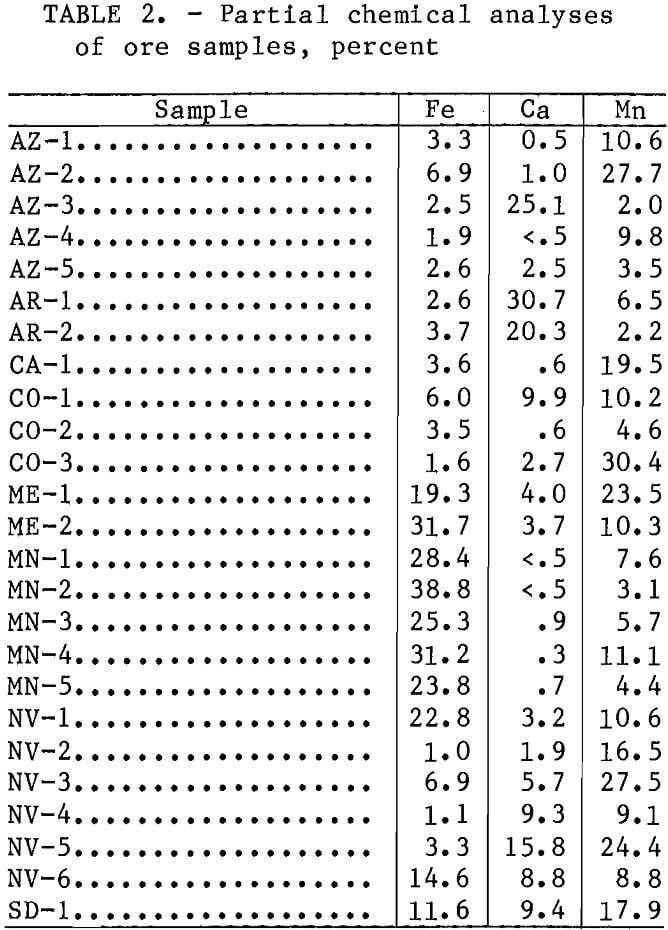
Each ore sample was ground to minus 2.5 cm and screened. Batch-leaching tests were conducted on the four finer size fractions, minus 0.841 mm plus 0.595 mm, minus 0.595 mm plus 0.210 mm, minus 0.210 plus 0.149 mm, and minus 0.149 mm, to determine optimum manganese extractions. Column-leaching tests were conducted on the minus 2.5- plus 1.3-cm size fraction of all ores. Initial col¬umn-leaching tests to determine the optimum solution application rate were made using a representative composite of the minus 2.5-cm plus 0.149-mm material of sample AZ-5.
Batch-leaching tests involved stirring 20 g of each of the four finer size head samples with 400 and 200 mL of a leach solution containing 6.4 wt pct SO2 and 200 mL of leach solutions containing 1.6, 3.2, and 4.8 wt pct SO2. These tests were conducted for 30 min at ambient temperature and pressure. The leach solutions were prepared by bubbling SO2 into distilled water, analyzing for SO2 content by iodometric titration, and then diluting to the final desired SO2 concentration. After completion of the leaching tests, the solutions were filtered and the residues were washed with distilled water. The filtrates and dried residues were analyzed for Mn, Fe, and Ca by atomic absorption spectroscopy. Metal extractions were calculated using both the filtrate and residue analyses.
Column-leaching tests were conducted in 10-cm-diam columns that were 1 m long and contained 3.5 kg of minus 2.5- plus 1.3-cm ore or mineral-sample pieces. A 5-wt-pct-SO2 solution was applied at a rate of 1 mL/min. This rate approximated the application rates in gold-silver and copper heap-leaching operations and resulted in an unsaturated column of ore. The tests were usually run until the Mn in the effluent from the column dropped to about 1 g/L. At least 50 L of solution were applied to each ore sample during each test, except for the AZ-5, AR-2, and MN-2 samples, for which only 30, 41, and 45 L were applied before the Mn in the effluent dropped to about 1 g/L.
Results and Discussion
Batch-Leaching Tests
Average batch-leaching test results for the four finer ore size fractions (minus 0.841 mm plus 0.595 mm, minus 0.595 mm plus 0.210 mm, minus 0.210 mm plus 0.149 mm, and minus 0,149 mm) are given in table 3. These results indicated that pyrolusite, psilomelane, and wad were readily leached, as average Mn extractions of 95 pct were obtained for ores in which these were the predominant Mn minerals. Ores containing predominately carbonate mineralization (ME-1, MN-1, and SD-1), manganite (MN-3 and NV-1), and hausmannite-braunite (AR-1 and AR-2) were not as readily leached; average Mn extractions of only 67, 77, and 79 pct, respectively, were obtained. Ores containing predominantly rhodonite were leached very little. The 15-pct-Mn extraction observed for sample CO-3 was owing primarily to the leaching of MnO2, which originated from a thin layer of oxidized mineralization on the Surface of all CO-3 ore pieces before they were
comminuted for the leaching tests. The 19-pct-Mn extraction observed for sample ME-2 was primarily due to the leaching of the rhodochrosite present in this ore. These batch-leaching tests also indicated that Mn was selectively leached with respect to Fe but not with respect to Ca. A weighted average of 76.1 pct of the Mn in the ores was leached, while only 4.8 pct of the Fe was coleaehed. A weighted average of 73.7 pct of all of the Ca was also coleaehed.
Column-Leaching Tests
Effect of Solution Application Rate on Mn Extraction
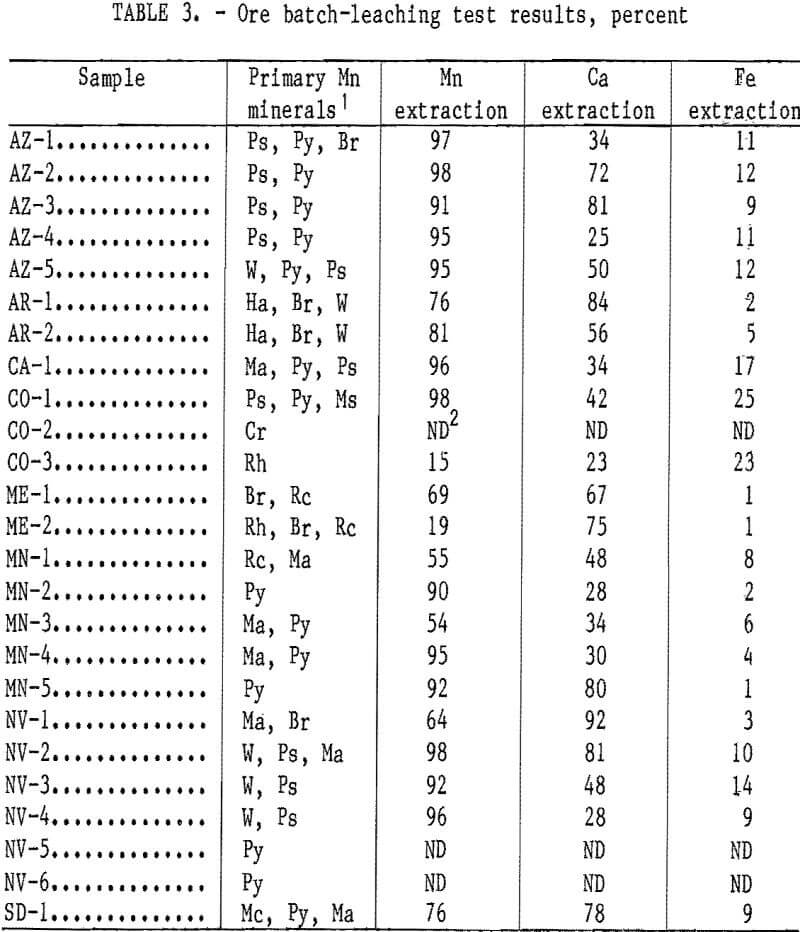
The effect of solution application rate on Mn extraction is shown in figure 1. For these tests, four 3.5-kg samples of AZ-5 ore representative of the minus 2.5-cm plus 0.149-mm size fractions were recombined and leached in the 10-cm-diam columns. The 5-wt-pct-SO2 solution was applied at rates of 18, 9, 5, and 1 mL/ min, and the percent Mn extraction determined as a function of volume of leach solution applied to the column. Application rates of 5 and 1 mL/min resulted in the greatest extraction of Mn in the least volume of solution. Apparently, at higher application rates, much of the leach solution cascaded through the column before it could enter the ore pieces to leach the internal Mn mineralization. Ca coextraction decreased from an average of 3.0 to 3.5 g/L to <0.5 g/L when the application rate was decreased from 18 mL/min to 1 mL/min. Fe extraction increased from an average of 0.2 g/L to 0.5 g/L when the application rate was decreased from 18 mL/min to 1 mL/min. Based upon the results of these tests, an application rate of 1 mL/min was chosen for subsequent testing.
Leaching of Mn Minerals
Figure 2 shows the percent extraction versus volume of solution applied curves for the Mn minerals pyrolusite, rhodochrosite, manganite, and rhodonite. For comparison, the highest Mn extractions obtained in batch leaching of the minus 0.841- plus 0.595-mm, minus 0.595- plus 0.210-mm, minus 0.210- plus 0.149-mm, or minus 0.149-mm size fractions of these minerals were 92, 7, 64, and <1 pct, respectively.
The column-leaching test results for pyrolusite and manganite were consistent with the batch-leaching test results. Both pyrolusite and manganite were leachable; however, the manganite-leaching reaction was slower. Leach solutions consistently containing about 3 g/L Mn were still being extracted when the manganite-leaching test was terminated.
The column-leaching results for rhodochrosite and rhodonite were not consistent with what was expected on the basis of the batch-leaching results. Apparently, the leaching of rhodochrosite in a batch mode results in a steady-state condition that Inhibits further Mn extraction. In the column-leaching tests,
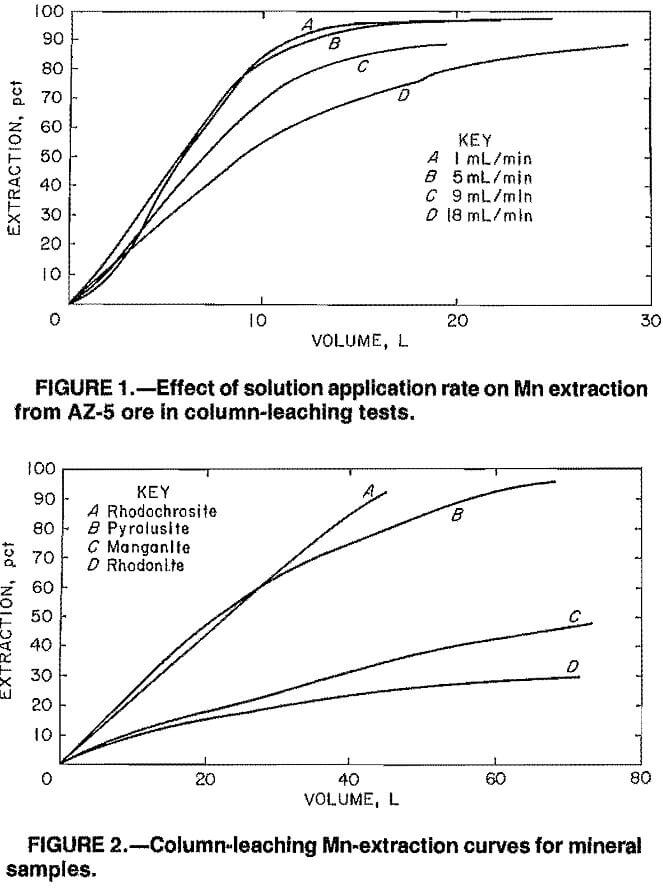
fresh SO2 solution was continually being applied to the column, and the steady-state condition was obviated. The result was that Mn was readily extracted in the column-leaching tests producing pregnant leach solutions averaging 30 g/L Mn. On the basis of the batch-leaching tests, one would conclude that extraction of Mn from rhodonite with SO2 solutions is not feasible. The column-leaching tests, however, indicate that leaching of rhodonite is possible even though the solubilization reaction is slow. Leach solutions consistently containing 1.5 to 2.0 g/L Mn were still being obtained when the test was terminated.
Leaching of Mn Ores
The ore column-leaching test results are given in table 4. Variations of Mn extraction with the volume of solution applied are shown graphically in figures 3-7. The teaching rate was greatest for the AZ-5 ore sample; however, higher or equal grades of effluent solution were obtained for the higher grade materials, AZ-2, CO-1, ME-1, NV-2, NV-3, NV-4, and SD-1. As in the batch-leaching tests, the ores containing primarily pyrolusite, psilomelane, and/or wad were readily leached. Mn extractions in the 80 to 95 pct range were obtained for these ores. The lower extractions in this range were generally owing to the termination of the tests while Mn extraction was still occurring.
In general, the batch test results for ores containing primarily manganite were similar to the column-leaching test results. Samples CA-1, MN-3, MN-4, and NV-1 all were leached at a slower rate.
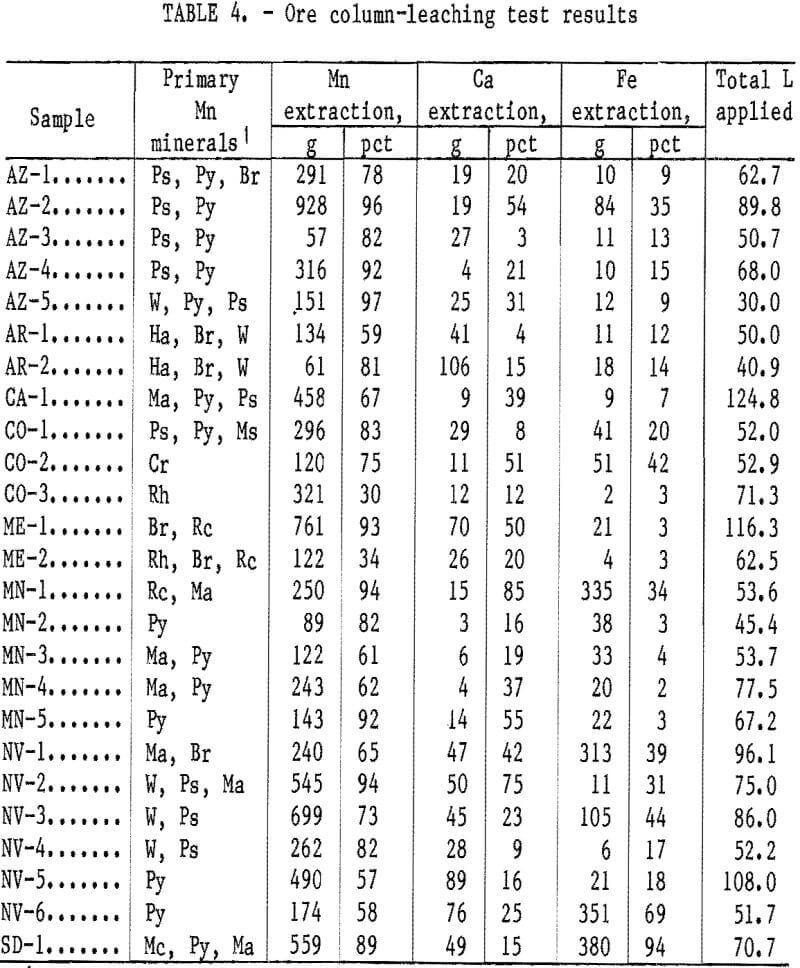
Mn extractions ranged from 60 to 70 pct for these ores.
Mn extractions for samples containing manganese carbonates (MnCO3) (MN-1, ME-1, and SD-1) ranged from 89 to 94 pct in column-leaching tests, compared to 55 to 76 pct in batch-leaching tests. The greater extractions obtained for column leaching of these ores, like those in column leaching of the rhodochrosite-mineral sample, were a result of nonsteady- state-equilibrium condition in the columns compared to the steady-state- equilibrium condition in the flasks.
Batch tests on the finer particles of ore indicated minimal Mn leaching from samples containing rhodonite (ME-2 and CO-3). The percolating action of SO2 solutions in column leaching, however, resulted in steady-state extractions from both ores of about 1 to 2 g/L Mn in the column effluent even after 62.5 and 70 L of leach solution, respectively, had been applied to the column of ore.
Cosolubilization of Ca and Fe
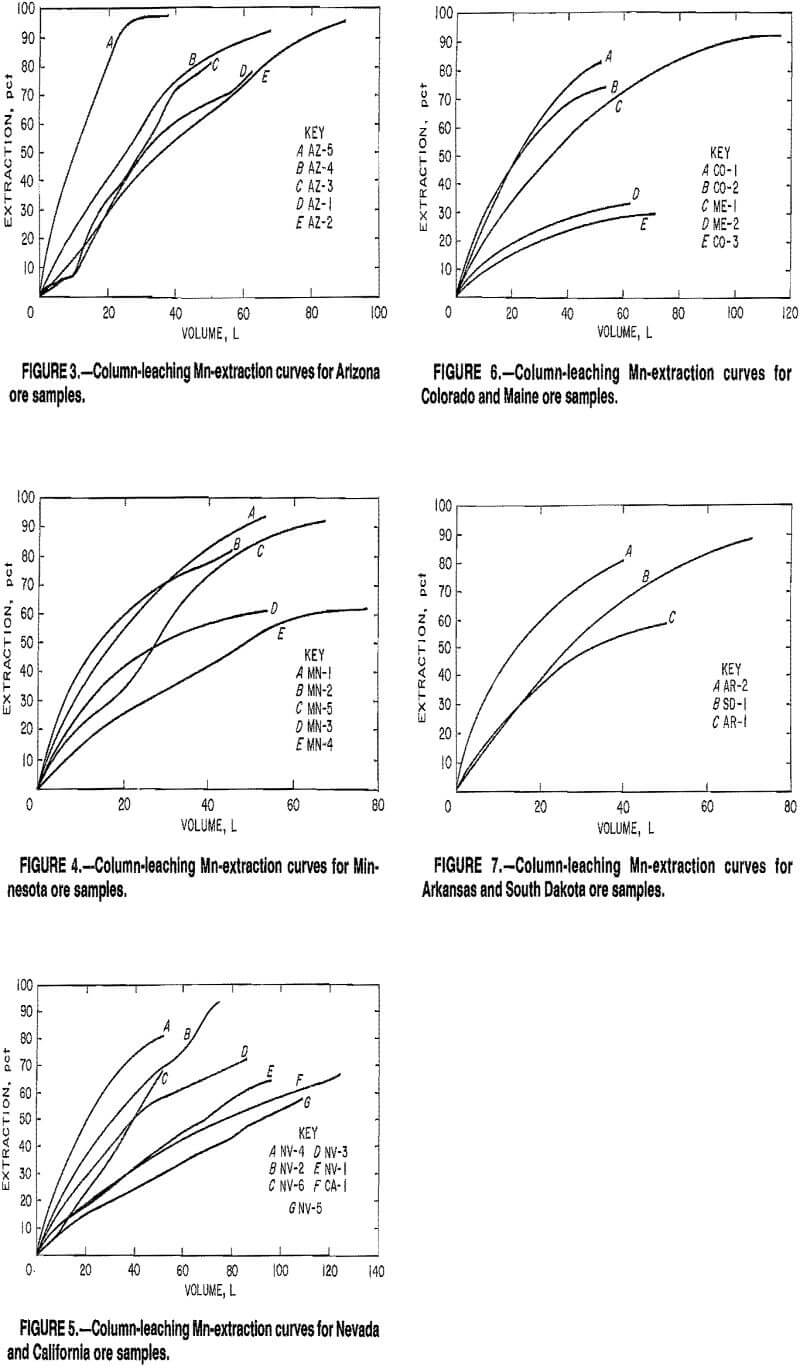
Mn can be selectively leached from ores containing Fe unless the Fe is present as a carbonate (siderite) or hydroxide (goethite). In any commercial-leaching operation for such ores, it would be necessary to employ a short leach time of a thin leach zone or small heap whereby most of the Mn is selectively removed from the whole ore sample before much Fe is leached. Figure 8 plots the weight of Fe extracted as a function of volume of solution applied to the column for ten ores that contained >10 pct Fe. The Fe in ore samples MN-2, MN-3, MN-4, MN-5, ME-1, and ME-2 was present as hematite. The Fe in ore sample NV-1, was present as goethite, while the Fe in ore samples SD-1 and NV-6 was present as siderite, and the Fe in ore sample MN-1 was present as a combination of goethite and siderite. Less than 3 pct of the Fe was cosolubilized with the Mn from the six hematite-containing ores, while 39, 94, 69, and 34 pct of the Fe was cosolubilized from ores NV-1, SD-1, NV-6, and MN-1, respectively. For the latter-type ores, Mn was selectively leached with respect to Fe as either goethite or
siderite from the top region of the column. However, with continual application of solution to this Mn-denuded area, Fe began to be extracted.
Weight ratios of Mn extraction to Fe extraction for column leaching of NV-1, SD-1, and MN-1 ores were 0.77, 1.47, and 0. 75, respectively. Corresponding weight ratios for batch leaching were 9.9, 13.1, and 1.8, respectively, showing that selectivity can be improved by leaching finer particles or by leaching for shorter times.
Although the reaction of SO2 with Ca is favored over that with Mn, Ca solubilization can be minimized by controlling the rate of solution application. It appeared that upon leaching the calcium carbonate (CaCO3), a CaSO3 layer was formed around the mineralization that inhibited further leaching. With a low solution-application rate (1 mL/min), the sulfite layer remained largely intact and Ca solubilization was kept at a minimum, as less than 0.5-g/L Ca was extracted from ore AZ-5 at this solution-application rate. Upon increasing the solution- application rate to 5, 9, and 18 mL/min, the sulfite layer was eroded to a progressively greater extent, and the Ca solubilization progressively increased, i. e., to 1, 2, and >3 g/L Ca extraction from ore AZ-5. Weight ratios of Mn extraction to Ca extraction for column leaching of the AZ-3, AR-1, C0-1, and NV-4 ores were 2.1, 3.3, 10.2, and 9.4, respectively. Corresponding ratios for batch leaching were 0.09, 0.19, 2.4, and 3.4, again indicating continual removal of the protective CaSO3 layer during agitated leaching.
In general, Mn ores with high CaCO3 contents can be percolation leached if the Mn minerals are not imbedded in the CaCO3 matrix and the rate of leach-solution application is low (about 1 mL/min). High Ca-extraction rates in leaching also lead to increased calcium sulfate (CaSO4) deposition throughout the ore bed. This also slows the rate of Mn extraction by preventing contact of the SO2 solution with the Mn minerals.
Induced Permeability
Most of the Mn ores employed in column leaching tests have low water permeabilities even though the porosity of the ores are not correspondingly low. For example, ore AZ-5 had water permeabilities of 10-² to 10 -4 darcy even though it had 23-pct porosity. From the standpoint of in situ or heap leaching, this ore would be considered nonleachable because of its low permeability. Microscopic examination of the ore showed that Mn precipitation resulted in closed capillaries, coated interiors of pores, and Mn deposits at matrix grain boundaries. Thus, upon leaching the Mn, the capillaries and pores are opened up and leach solution is allowed to ingress toward the center of the ore pieces to leach more Mn. This induced permeability made it possible to leach the largest pieces of AZ—5 ore tested, which were about 10 cm in diameter. This property of Mn ores should help minimize solution excursions during leaching of unsaturated deposits.
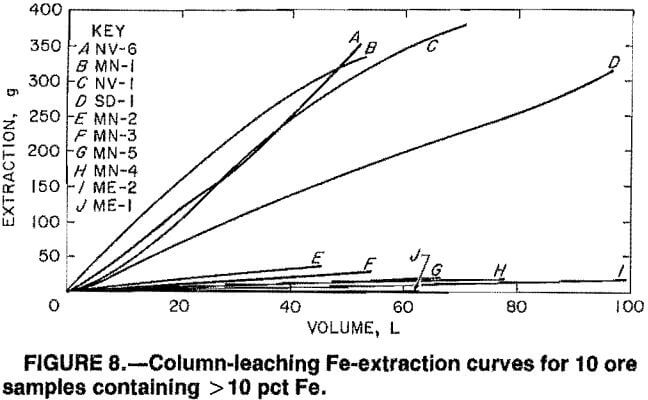
The solubilization reaction between SO2 and Mn was found to be rapid in previous work on sea nodules and in the present batch-leaching tests on fine ore particles. The rate of leaching, therefore, appears to be dependent upon the rates of pore diffusion of (1) the SO2 solution into the ore pieces and (2) the reaction products out of the ore pieces. The linearity of plots 1n[(1+f)/(1-f)] versus time for SO2 leaching of the ores is exemplified in figure 9 for ores AZ-4, CO-1, ME-1, NV-1, NV-5, NV-6, and SD-1 and is evidenced in table 5 for all 25 ores by the near unity correlation coefficients for a linear fit of the data. This proves that the rate data follow the rate expression for autocatalytic reactions
kt = 1n[(1+f)/(1-f)],
where k = rate constant,
t = time,
and f = fraction of Mn leached at time t.
Since the reaction of SO2 solution and Mn has been observed to be rapid for Mn4+ oxide and hydroxide species, the limiting rate of leaching is the rate of solution ingress and reaction-product egress.

Conclusions
The reliance of the United States on foreign sources of supply for 98 pct of the Mn it consumes yearly has stimulated interest in new and innovative methods of producing Mn from small and/or low-grade domestic deposits. One method that appears to have promise for fulfilling some nonferromanganese requirements is in situ and/or heap leaching.
Column-leaching tests were conducted with 5-wt~pct-aqueous-SO2 solutions on 25 samples from 14 mining districts in eight States to simulate in situ- and heap-leaching reactions. Mn contents of these ores ranged from 2 to 30 pct. Column-leaching of 11 ore samples containing primarily pyrolusite, psilomelane, and/or wad resulted in 73- to 97-pct Mn extraction, with an average extraction of 86 pct. Column leaching of three ore samples containing primarily manganese carbonates, two ore samples containing primarily hausmannite-braunite, four manganite-containing samples, and two rhodonite-containing samples resulted in Mn extractions of 89 to 94, 59 to 81, 61 to 67, and 30 to 34 pet Mn, respectively, with average extractions of 92, 70, 64, and 32 pct.
By limiting the SO2 in solution to 5 wt pct and controlling the solution application rate, Mn can be selectively leached from Fe unless the Fe is present as goethite or siderite. Injection of the leach solution at progressively lower points in heap leaching may help lower the Fe coextraction. Ca solubilization can be inhibited by controlling the solution-application rate in order to prevent eroding of a protective CaSO3 layer on the Ca mineralization.
The rate of Mn solubilization was observed to be rapid, and, therefore, the overall leaching rate was found to be dependent upon the rates of solution ingress into the ore pieces and reaction- product egress out of the ore pieces. Mn ores initially have low permeabilities but are leachable since permeability is induced as leaching progresses.
It appears that Mn ores containing pyrolusite, psilomelane, wad, hausmannite, braunite, manganite, rhodochrosite, and even rhodonite may be in situ or heap leached without prior calcining, as was done by previous researchers. Addition of H2SO4 to S02 leaching solutions, which was also done by previous researchers, is not needed in the leaching of Mn-containing ores. Pyrolusite, psilomelane, wad, and rhodochrosite are readily leached by applying 5-wt-pct-SO2 solutions at a rate of 1 mL/min, while more time and solution are required for leaching of manganite-, hausmannite-, and braunite-containing ores.
