Table of Contents
When the cyanide process was first introduced its application was confined to leaching, that is, treatment by percolation. By this method only such material could be worked as was sufficiently granular in texture to allow the necessary amount of solution to percolate through it in a reasonable time. By crushing the ore dry, it is sometimes possible to leach it direct without removing the slime, but in the majority of cases this is not feasible, and it is necessary to separate the sand from the slime, leaching only the former and treating the latter by agitation.
Separation of Sand and Slime
In cyaniding the current tailing produced by amalgamating or concentrating mills, a rough separation of the percolable material was originally made by running the pulp into pits where the sandy part was deposited, allowing the slime to overflow and either letting it go to waste or settling it in large dams from which the clear water could be pumped back to the mill.
Later, a system was developed whereby the pulp from the mill was led to collecting tanks, in which the sand was retained and the slime flowed away, not yet to any method of treatment but to storage dams.
There were two methods in use at this time in South Africa for effecting the separation of the sand, one was by the Butters and Mein distributor, and the other by the “nigger with a hose.”
The Butters and Mein distributor consists of a circular hopper from which radiate pipes of various lengths and diameters, whose extremities are bent horizontally at a right angle, and sometimes flattened to produce a fan-shaped discharge. The length and diameter of each pipe is so calculated as to deliver an equal tonnage of pulp to a proportional ring-area of the tank. The hopper is supported or suspended level with the top of a circular tank, on a bearing, so that when the stream is led into it the discharge from the pipes causes the whole to revolve slowly, with the object of making a uniform deposit of sand over the surface of the tank bottom.

In the original form of this machine the tank was fitted with an annular overflow launder, and was filled to the brim with water before the pulp was turned into it. When in action, the sand sank down to the bottom, and the slime and surplus water overflowed into the ring-launder until the tank was filled with sand. The drawback to this system as a separator of sand and slime was that in the event of a shut-down of the mill all the slime in suspension above the sand settled down on it forming an impervious layer. The machine itself, however, has come back into favor as a method of filling a tank with sand from which the slime has been previously separated, a function which it performs very satisfactorily.
An improved form of this machine, when used as a separator, consists in discarding the top overflow and making two or more openings, capable of being closed by water-tight doors, in the side of the tank near the bottom. On the inner side of the tank at the point where the doors are placed an adjustable weir is attached, whose height of discharge can be raised as the level of the sand rises, either by slipping narrow wooden slats into a pair of grooves, or by rolling up a canvas curtain over a wooden grating. This system works fairly well, but has a tendency to leave groove-shaped depressions containing solid deposits of slime.
With the “Nigger and Hose” system the pulp is led to a large spitzkasten or spitzlutte where part of the slime and water overflows; the remainder or underflow discharges into a large rubber hose leading to the sand tank to be filled. The tank, in this system as in the one already described, is fitted with low exit doors and adjustable weirs for the removal of the slime and water. A Kaffir is stationed in the tank to keep the discharge end of the hose moving from place to place so as to build up a deposit of sand as evenly and as free from slime, as possible.
The quality of the charges produced by both these methods is fairly satisfactory, but varies within wide limits according to the diligence and efficiency of the shift-man. It is found difficult, however, to get a satisfactory direct treatment of such charges, owing to the density of packing and inhomogeneous nature of the deposit, and they are almost invariably transferred to another tank for the leaching treatment.
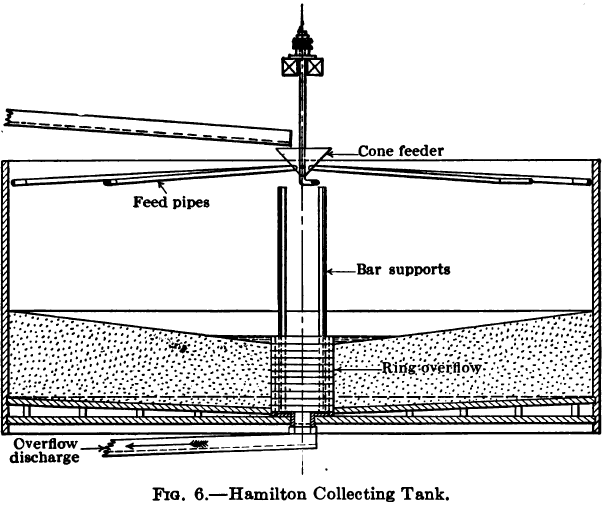
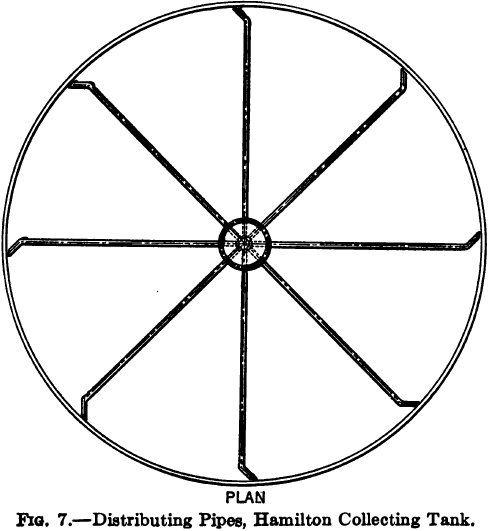
Hamilton Collecting Tank
To obviate the erratic deposition resulting from the Butters and Mein distributor, and to combine the advantage of the hose distribution with that of automatic and mechanical efficiency, the writer designed a collecting tank in which the pulp entered at the periphery and the slime flowed off at the centre. The intake of pulp was effected by a mechanism similar to the Butters and Mein distributor except that all the pipes were of the same length and extended almost to the side of the tank, being bent at their extremities not at a right angle but at 45 deg. so that the pulp would discharge against the side of the tank without making any splash and yet exert sufficient tangential force to cause the hopper to revolve on its ball bearing.
The central overflow weir was circular and its height was adjusted to the rising level of the sand deposit by adding, periodically, tongued and grooved rings 1½ to 2 inches in height and thus gradually building up the discharge level until the tank was full of sand.
During filling the pulp was thrown uniformly against the side of the tank whence it flowed down vertically to the bottom, and then toward the centre discharge, the sandy portion building up in the form of an inverted cone, sloping toward the centre, and the slime and water flowing over the centre weir. In working, the sand surface was never allowed to become submerged except for a few inches around the central overflow, so that the sand particles could settle down one on another leaving the slime to flow on over the surface till it reached the small tranquil pool just around the overflow which served to trap and hold the finest of the sand. Very little slime was deposited in this pool because as the film of water and slime flowing over the inclined surface converged toward the centre the velocity increased sufficiently to hold the slime in suspension until it had passed over the weir. The tank was fitted with a filter bottom of the usual construction but coned slightly toward the centre, for draining the charge dry when collected.
This system proved a great improvement over previous methods especially in handling the fine material resulting from tube mill regrinding, being capable of separating sand, a large proportion of which was finer than 150 mesh and yielding a leaching product containing only from 4% to 5% of slime uniformly distributed through it. The following table gives a screen analysis of sand charges collected by this system at a mill where really fine regrinding was practised. This material when transferred to the leaching tanks percolated at the rate of 1½ inches an hour through charges 3 ft. in depth.

Double Treatment
In the course of experiments in direct treatment it was found that when a charge was moistened with cyanide solution before being transferred to the treatment tank the extraction was invariably better than when the first contact with cyanide took place after transferring, and this was attributed to the increased activity of the solution caused by the exposure of the cyanide-coated particles of ore to the action of the air in transferring. This discovery led to the universal adoption on the Rand of the double treatment system of leaching, which was the standard practice for many years and is largely made use of up to the present time (1919).
At the Homestake mine where the assay value of the original sand was so low as to make economy in handling absolutely necessary to a commercially profitable treatment C. W. Merrill devised a separating system whereby the double treatment was rendered unnecessary. The principle involved was a complete separation of the slime before collecting the sand leaching charge, and this was effected by means of a series of Merrill Cones. This series is composed essentially of two sets of gravity cones followed by one set of smaller hydraulic cones, the underflow of each set forming the feed of the next set in the series.
The underflow or finished product from the hydraulic cones passes to a Butters and Mein distributor tank of the original type with peripheral overflow and the charge after collection is so homogeneous and porous that leaching can be completed without any transfer. The aeration, which proved to be an indispensable adjunct to the successful treatment of this ore, is effected by draining the charge dry and introducing compressed air under the filter bottom for a set period of time before applying the first cyanide wash. The aeration is repeated at intervals during treatment, between solution washes.
Caldecott’s Choke-discharge Cone
For making a clean separation of the sand Caldecott uses a cone with a metal disc or diaphragm fixed on the inside near the apex. This disc may have a plain or serrated edge and is so placed as to leave an annular space between its edge and the side of the cone. At the apex is a plug cock or other device for regulating the rate of discharge. When working, the latter is regulated so as to maintain a solid bed of settled sand extending up to within 10 in. of the overflow. Under these conditions a thick sluggish stream of almost clean sand issues from the bottom while the water and suspended slime overflows around the top. The purpose of the diaphragm is to minimize the chances of a funnel forming in the bed of sand, through which the slimy water would break and issue from the spigot.
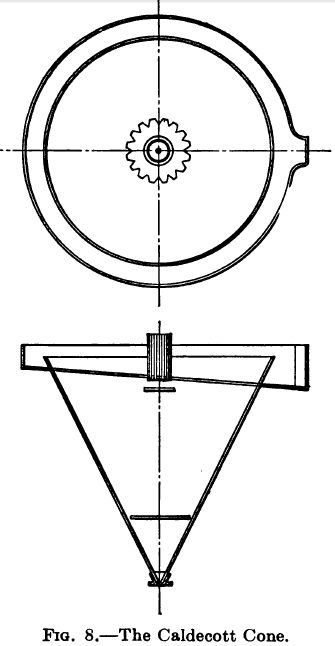
As a certain amount of slime is entangled with the sand produced in this way, Caldecott recommends passing the product to a second diaphragm cone with clean water to complete the separation.
The sand from this second cone is run onto a revolving horizontal vacuum filter where the residual moisture is reduced to a minimum. At the completion of each revolution of the table the drained sand is automatically scraped off into a hopper where it meets a flow of cyanide solution, and the pulp thus formed is pumped to a Butters and Mein Collecting tank with peripheral overflow, as at the Homestake.
The advantage of the Caldecott cone over the Merrill seems to be in the possibility of a more complete elimination of the slime, because in the latter the spigot discharge is free and it would seem inevitable that more slime should escape with the finished product than would be the case with a choke discharge. When milling is done in water the Caldecott system also permits of pulping the sand in cyanide solution before collecting the leaching charge, and thus lengthening the time available for contact.
Both these systems of sand collection utilize the earlier form of Butters and Mein distributor tank, but with the important difference that this apparatus is used exclusively as a collector and not as a separator of sand from slime. The tank with its annular overflow is filled with water or cyanide solution and the clean separated sand is fed to the distributing hopper in a medium of water or solution, the solids gradually replacing the liquid until the tank is filled with sand.
The two main results accomplished by this change are first, the impossibility of collecting a slimy charge provided that the previous separation has been properly conducted, and second, the formation of a sand charge of such uniform texture and percolating properties that the whole leaching treatment can be carried out in the same tank, yielding the maximum extraction obtainable and obviating the cost of a transfer to another tank during the treatment.
A charge collected in this way possesses superior uniformity of structure and leaching and aerating qualities to one collected by any of the methods using an adjustable overflow weir which keeps the sand surface only partly submerged, on account of the different positions assumed by the individual particles relatively to one another in the two cases. When angular particles in a water medium are rolled over one another or down an incline composed of similar particles there is a strong tendency for them to come to rest and find lodgment with their flat surfaces in contact with one another, forming an interlocking and compact mass with a minimum of interstitial spaces, whereas when they sink slowly through a tranquil body of water they are likely to remain in the relative positions in which they first come to rest and that will be, as often as not, with angles in contact with plane surfaces, giving more interstitial capacity and therefore making the whole charge more permeable to solution and to air.
By this method a uniform mixture of coarse and fine particles is also attained, and the deposition of patches, bands, and strata of alternating coarse and fine material is avoided, thus allowing of a uniform rate of percolation through every part of the charge.
The introduction of mechanical classifiers such as the Dorr, Esperanza, Bayliss, Colbath, Akins, etc., affords a very convenient and efficient method of applying this system.
In principle, of course, nothing could be simpler than a cone classifier, but in practice it needs constant attention to avoid irregularities in working. Bits of wood, waste, washers, nuts, and various other articles have a way of inserting themselves in the apex and if not at once seen and removed, throw that unit out of commission for the time being. Hydraulic cones have a tendency, too, to bank up gradually, even when the spigot discharge is free and unobstructed, and generally require emptying at intervals.
The proper working of the Caldecott cone, also, depends on a close adjustment of the spigot discharge to the amount of inflowing pulp. Any falling off in the quantity of sand entering causes a lowering of the sand level within the cone and finally a “ breaking through ” or discharge of muddy water through the spigot, while any sudden increase in the pulp supply raises the sand level in the cone to a point where sand is carried over in the slime product. This fluctuation is not so important when working with large units which allow of a fair margin of variation, but with small units constant attention is needed in order to obtain satisfactory work.
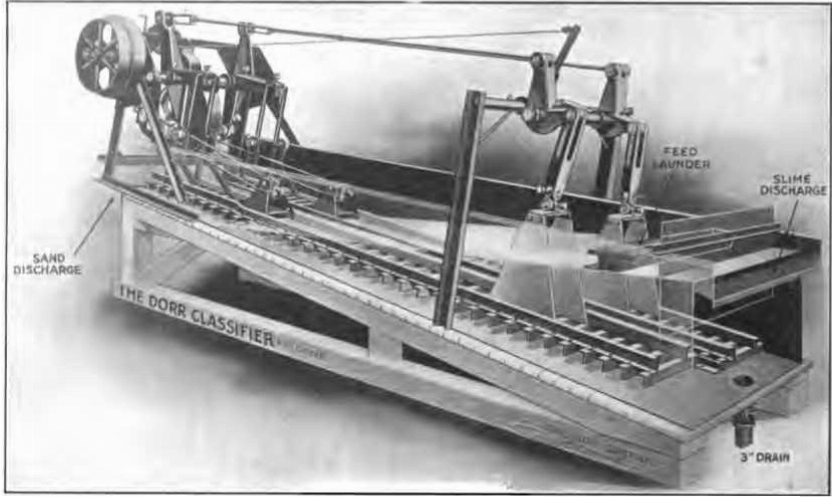 Fig.9 The Dorr Classifier
Fig.9 The Dorr Classifier
The mechanical classifier, on the other hand, provided that it is not overloaded and that the ratio of liquid to solid is sufficiently large to allow of the disentanglement of the sand from the slime by the action of gravity, will perform its function efficiently regardless of wide variations in pulp flow, and with practically no attention.
A very satisfactory system of sand leaching by direct treatment in use in at least one Mexican mill consists in separating the sand in a mechanical classifier, adding clear solution to the product and passing it to a second similar classifier, and then transferring from this by means of a stream of clear solution to a Butters distributor tank with peripheral overflow. When the tank is full of sand the leaching treatment is proceeded with and completed in the same tank, and the residue sluiced out of the dump.
A useful form of classifier for thoroughly washing the sand free from slime before discharging it into the Butters distributor for this system of collection would seem to be the Dorr washing classifier which combines three machines in one, the first compartment separating the sand from the slimy water or solution and discharging it successively into a second and third compartment containing clear solution which completes the removal of the entangled slime.
How to Leach Gold
Leaching is usually carried out in circular tanks of wood or steel with a false bottom covered with some filtering medium. A grid is first made on the bottom of the tank by laying wooden strips about 2 in. by 3 in. on edge, a foot apart, and nailing across and on top of these, smaller strips having a square section of 1 to 1½ in., and leaving 1-in. spaces between them. This grid is preferably made in sections sufficiently small to be handled by two men, as it has to be lifted periodically for the purpose of cleaning out accumulated sand and slime that may have worked through the filter. On top of the grid is laid a circular mat usually composed of ordinary cocoa fibre matting, and on this a cloth of a texture fine enough to prevent the passage through it of slime and fine sand. The best material to use for this purpose is closely woven jute cloth such as is used in Mexico and Central America for sacking coffee for export. The material commonly known as “burlap” is not suitable as a top covering because the texture is usually too coarse. Six- or eight-ounce duck is often placed above the cocoa mat instead of jute and answers the purpose well except that it tends to reduce the rate of percolation considerably.
The top covering, whatever it may be, is securely fastened in contact with the side of the tank, either by extending it a few inches up and tacking a flexible strip of wood over it, or in the case of a steel tank by driving a thick rope with a wooden caulking tool down between the grid and the side of the tank. For this purpose the grid is made 2 in. smaller in diameter than the size of the tank, and is enclosed by a continuous rim made from a flexible strip of wood, so as to give a solid backing for the rope caulking.
A pipe, 1½ to 2 in. in diameter, enters the tank through the bottom for drawing off the solution. The system of connecting the outflow pipes from the whole system of leaching tanks into a single header for conveyance to the precipitating house is not recommended, as it makes control of individual charges difficult both as regards sampling the effluent solution and also adjusting its rate of flow. If the precipitation house is at a distance from the leaching tanks, the individual pipes may discharge through regulating valves into a small tank or box near by, from which the solution may flow in a large common pipe fine to the extractor house. If it is desired to circulate two different grades of solution, the small receiving tank may have two compartments, and the solution from any tank may be readily diverted to either compartment.
When the sand tank is filled and ready for treatment the solution may be run onto the surface of the charge and allowed to percolate downward, or it may be introduced under pressure beneath the filter and caused to percolate upward. The latter method is chiefly valuable where the charge contains a large admixture of fine material and slime, such as results when a dam of accumulated tailings is dried and coarse and fine mixed together for treatment of the whole by percolation. Such a charge might pack so densely under the influence of gravity percolation, especially if drained dry after a wash, that no subsequent washes would pass through, whereas by continuous upward percolation and removal of the effluent from the surface a rapid rate of flow may be maintained throughout the treatment. The usual practice, however, is to apply the solution on top and let it gravitate downward through the charge. It is often found a good plan to fix a stand pipe of about two inches in diameter against the side of the tank, the upper end terminating at the top and the lower end extending down through the filter mat which is tightly caulked around it. This acts as a vent for the air driven down in front of the mass of descending solution, which would otherwise be forced to escape upward through the sand in the form of bubbles, thus forming channels and spoiling the uniformity of the subsequent percolation. The leaching cock is better kept closed until the whole charge is saturated and the solution stands permanently above the level of the sand. It may then be opened and regulated to any desired rate of flow.
When the cyanide process was first introduced a great point was made of letting the solution remain in contact for many hours before drawing off. This soaking procedure does not seem to have anything to recommend it, since diffusion is almost impossible during this stagnation period, and consequently the film of solution in contact with the particles would soon become saturated, and also denuded of its oxygen, with the result of delaying rather than hastening the dissolution of the precious metals. The idea underlying this practice was probably the desire to effect the dissolving with as small a bulk of strong solution as possible, both as a means of saving cyanide and also of obtaining a solution richer in the precious metals than would otherwise be the case. A method of accomplishing this result without retarding the dissolving action has been used with good results in some mills, notably at Kalgurli, and El Oro, Mexico.
This consists in attaching a small air lift to the outside of the tank, arranged so that as the solution percolates and reaches the space beneath the filter bottom it is at once returned to the surface of the sand. In this way a small bulk of strong solution is kept circulating through the charge for as long as maybe deemed advisable, and in its passage is given an opportunity of taking up oxygen to replace that which has been absorbed by the ore.
There are two methods in use for applying the solution. The first is to pump it on as fast as it percolates, thus keeping the charge always saturated or even covered, and the second is to pump on enough to saturate the charge and cover it to a depth several inches and then to drain it off and allow the sand to stand dry for several hours before the next application of solution. The second method is almost always preferable, because, except in the case of highly oxidized gold ores, the circulating solution does not carry sufficient dissolved oxygen to satisfy the reducing action of the charge and needs to be supplemented by other opportunities for oxygen absorption. By draining dry between washes the air is drawn down into the interstices of the charge as the solution recedes, and finds ideal conditions for absorption. In the case of very fine sand it is useful to apply a vacuum under the filter to remove the excess moisture due to the increased action of capillarity. When dealing with exceptionally reducing ores compressed air may then be introduced beneath the filter, if necessary, as is done at the Homestake. The system of draining dry between washes as compared with continuous leaching has been known to result in an increased extraction of 25% under actual working conditions on combined gold and silver ore.
The rate of percolation is usually measured by the number of inches per hour that the level of the solution on top of the sand falls as the wash percolates downward and flows out through the discharge pipe. Generally speaking the finer the sand the slower is the rate of percolation, but Julian and Smart state that “if an ore be crushed to pass through an ordinary assayer’s sieve of 90 mesh and washed free of slime, a good leachable product may be obtained, but if this fine ore be mixed with coarser particles, percolation becomes slower than the average of the two sizes treated separately.” The presence of slime, even when uniformly distributed, has a serious retarding action on the rate of percolation, especially when leaching fine grades of sand. The depth of the charge is also a factor to be considered, there being a slight retardation in percolation for every additional foot in depth. As regards standard rates of percolation 3 inches and over per hour is considered good, 1½ inches fair, and ¾ inch bad.
Retained Moisture
The finer the sand the more moisture is retained in the pores of the charge, but even with comparatively coarse sand there is a zone at the bottom that will never drain even approximately dry. This zone or layer is usually about 6 inches in depth regardless of the depth of the tank, and would therefore suggest the superiority of deep tanks over shallow ones, but against that is to be set the decreased rate of percolation as already noted and also the superior opportunities for aeration in a shallow charge. This wet layer at the bottom can be dried and aerated by the application of a vacuum under the filter after the solution has ceased to percolate by gravity. A vacuum used in this way is especially advantageous in the leaching of clean fine sand, which although it admits of rapid percolation by gravity yet retains a disproportionate amount of moisture which can only be displaced by pressure.
An important point in securing good leaching is to see that the mats and filter bottom are kept clean and thoroughly pervious to the solution. Sometimes the fine upper cloth may take on a deposit of lime carbonate, especially if aeration by compressed air is made use of, and in that case it will be necessary to clean it with dilute muriatic acid. Even under ordinary conditions the mats and the spaces underneath them tend in time to get blocked up with fine slimy material and require periodical cleansing.
The quantity of solution used during treatment varies from ¾ to 1½ times the weight of ore, according to circumstances, and has to be determined by the metallurgist from economical tests.
The solution strength is on the whole usually higher than is used in agitation processes, and various strengths are used on the same charge. When treating accumulated tailings, the common practice is to begin with one or two weak washes so that the cyanicides may expend themselves before the stronger solution is applied. With freshly crushed ore, especially if milling or collecting be done in cyanide solution, this is usually unnecessary and strong solution may be added at once, with the advantage of saving time and beginning the dissolving action as early as possible. The strength of this solution varies, but a concentration of 0.3% KCN or 6 pounds per ton of solution is probably used more often than any other.
The cyanide necessary to make up the stock solution to the desired strength is best added before the wash is applied to the sand tank. It is a common practice to hang cans containing lumps of cyanide in the stream of solution as it flows onto the sand, but this method is not recommended as it cannot produce a wash of uniform strength.
The most approved plan is to keep a solution tank expressly for strong w ashes. The solution therein is tested once or twice a day and the necessary quantity of cyanide placed in a basket hanging below the surface. When the lumps are all dissolved the contents of the tank are well mixed either by blowing compressed air into it for a short time or by circulating with a centrifugal pump. The resulting strong solution can then be pumped or run by gravity onto the sand charges needing it.
Draining and Washing
When all the soluble precious metal has been dissolved the strong rich solution in the charge is displaced by repeated washes of weak barren solution and finally by a water wash. Julian and Smart’s experiments go to show that given a certain volume of wash solution available its displacing efficiency is considerably increased by dividing it up into a number of small washes rather than applying the whole of it at once.
The quantity of water given at the finish is determined by the amount of moisture held in the charge when drained and ready to be sent to the dump, because although it would be desirable to give sufficient to displace the whole of the cyanide solution held as moisture, this usually could not be done without increasing the total quantity of solution in stock, with the result that the surplus would have to be run to waste, consequently only such an amount of water is given as is just equal to the quantity of moisture sent out of the plant in the residue.
It is advisable to assay the effluent solutions frequently throughout the treatment, especially during the washing-out stage of the process so as to observe the progress of displacement of the value-bearing liquor and avoid losing dissolved gold in the residues.
Discharging the sand is done in various ways. Where water is plentiful sluicing is a cheap and effective method. If this is not available, the tanks are so constructed that cars may be run underneath and the residue shovelled into them through doors in the tank bottom. Where labor is expensive, the Blaisdell excavating machinery in conjunction with a conveyor belt and stacker is probably the most satisfactory way of disposing of the residue.
Hamilton
