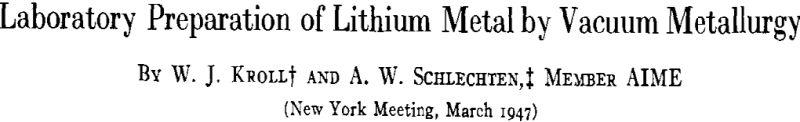Table of Contents
As this paper is written, the only method for the commercial production of lithium metal is by the fusion electrolysis of LiCl-KCl mixtures, as first proposed by Guntz. The details of the industrial process have not been made public but Osborg stated that an efficiency of more than 90 pct is obtained with a lithium metal recovery above 95 pct and that the metal is 99.5 pct pure. Pletenev gave a power consumption of 66 kw-hr per kilogram of lithium metal produced with a salt consumption of 9 kg of LiCl and 0.4 kg of KCl. Pletenev’s figures indicate a somewhat lower recovery than that given by Osborg. In a recent report on German lithium plants, Motock gives figures that are also less favorable than those reported by Osborg. At the German plants the lithium recovery in electrolysis was only 83.4 pct, with a power consumption of 140 kw-hr per kilogram of lithium. The metal averaged only 97 pct pure, with sodium and potassium as the chief impurities.
Previous Work
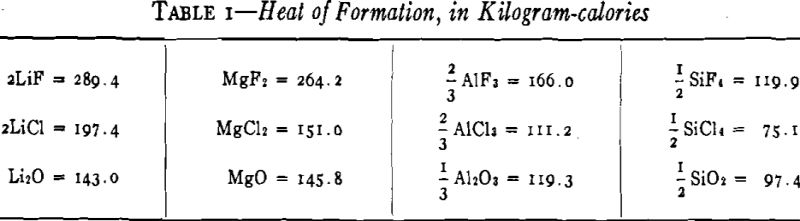
Various proposals have been made for thermal reduction of lithium compounds. In Gmelin it is stated that Warren reduced LiOH with magnesium, that Hackspill used calcium to reduce LiCl, and that Troost suggested sodium as a reducing agent for LiCl.
There are serious objections to all of these combinations. The reduction of LiOH with magnesium is violent, and the product is poor because hydrogen evolved by the reaction recombines with the lithium in the condenser. The volatility of magnesium is so nearly that of lithium that clean separation of the two metals appears to be impossible. The use of calcium as a reducing agent is hardly commercially feasible because of the high cost and high atomic weight of this metal. The reaction of lithium chloride and sodium rapidly reaches an equilibrium, with the production of very little lithium; high concentration of LiCl must be maintained, and the depleted salts must be reclaimed.
Experimental Equipment and Procedure
The vacuum furnace used for the experiments described in this paper consisted of a porcelain tube 40 in. long with an inner diameter of 2 1/8 in. The tube was supported vertically with the closed end on top; a metal head was fastened by a wax seal to the lower end of the tube. Vacuum connections were made through the metal head, which also had a vacuum-tight cover with a conical ground joint that could be opened for the introduction of samples into the tube. A mechanical pump in series with an oil-diffusion pump gave a vacuum of less than one micron in the system. The vacuum was measured with a Stokes tilting McLeod gauge.
Production of Lithium Oxide
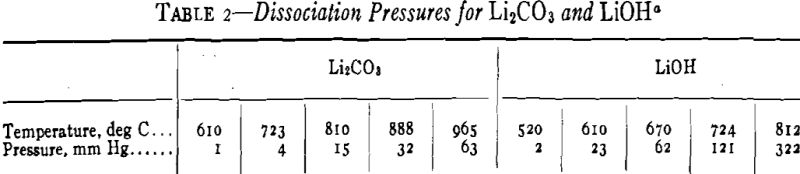
Lithium oxide is not commercially available, but it can be produced by thermal decomposition of the carbonate or the hydroxide. Johnston gives dissociation pressures for the two compounds (Table 2).
The melting point of Li2CO3 is 732°C and that of LiOH is 455°C. They reach a dissociation pressure of 760 mm Hg at 1270°C and 924°C, respectively. Avail-able data indicate that Li2CO3 is dissociated more easily than BaCO3 but less readily than CaCO3.
Few data are available on the physical and chemical properties of Li2O. It is said to be slightly volatile at 1000°C and to melt above 1625°C.
Reduction of Lithium Oxide
The reduction of mixtures of lithium oxide and calcium oxide with silicon, by analogy with the similar reduction of dolomite with ferrosilicon, probably proceeds according to the following equation:
2Li2O + 2CaO + Si = SiO2.2CaO + 4Li……………………………………………..[1]
The ground mixture of Li2O-CaO from the calcination described in the previous section was thoroughly mixed with finely pulverized silicon (100-mesh). A 10 pct excess of silicon was added over that indicated by Eq 1. The mixture was briquetted and heated in the vacuum furnace under a residual pressure of one micron or less. The furnace was held for 15 hr at the maximum temperature. The deposition of lithium metal in a ring in the cooler part of the furnace immediately below the heated zone could be observed through the small window at the lower end of the tube.
An average sample of lithium metal made by the silicon reduction of a mixture of lithium oxide and calcium oxide in four different runs contained: 0.01 Si, 0.01 pct Al, and 0.04 pct Ca.
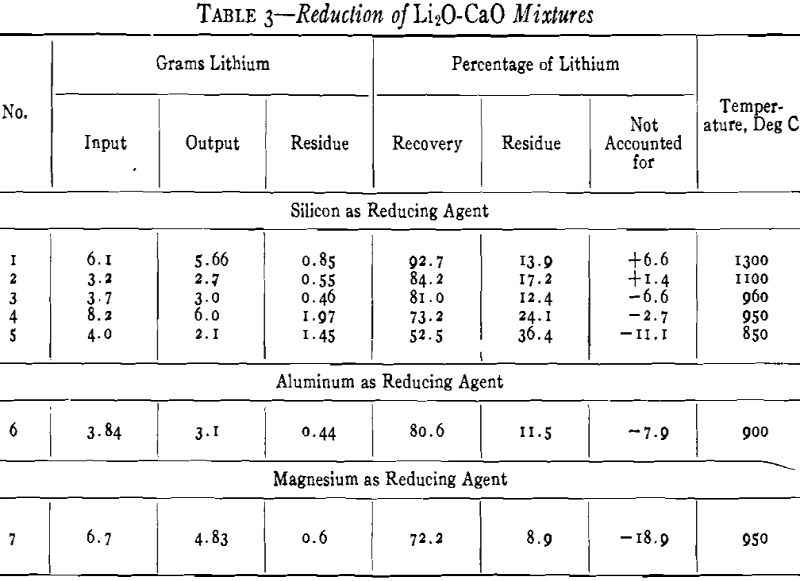
Reduction with Aluminum
The reduction of lithium oxide with aluminum resembles very closely the reduction with silicon. The following equation represents the probable reaction:
3Li2O + CaO + 2Al = CaO.Al2O3 + 6Li…………………………………[2]
The experimental procedure was the same as that used with silicon; a 10 pct excess of aluminum above the theoretical amount required was added to the Li2O-CaO mixture.
At a temperature of 900°C and under a vacuum of one micron or less, 80 pct of the lithium in the charge was recovered as metallic lithium.
It appears that approximately the same recovery is obtained with aluminum for the reducing agent as with silicon, but slightly lower temperatures can be used.
Reduction of Lithium Chloride
A process has been developed by one of the authors for producing metallic sodium from NaCl according to the following equation:
4NaCl + 4CaO + Si = SiO2.2CaO + 4Na + 2CaCl2………………………………….[3]
In this operation sodium is evaporated because it is more volatile than its chloride; the boiling points are, respectively, 850° and 1685°C. The margin of operation with lithium is not nearly as great; the metal boils at 1257°C and LiCl at 1384°C. Silicon can be replaced by aluminum.