Table of Contents
In a broad sense, sulfide copper pyrometallurgy is a batch sequence in three separate vessels, i.e., reverberatory furnace to yield “matte copper,” a converter to produce “blister copper,” and another reverberatory furnace to produce fire-refined copper. Matte smelting is relatively unchanged since it became universal practice some 50 to 60 years ago. It is now serving wide range of smelter feed materials, but many concentrates ore not immediately amenable to the process. There may be an excess or deficiency of essential matte-forming, elements, so adjustments must be made to the furnace charge. When smelter feed carries a sufficient excess of sulfur, byproduct manufacturing of elemental sulfur or H2SO4, may be incorporated into the metallurgical flowsheet. At the other extreme, there arc abundant copper resources which lack sufficient sulfur and other elements to sustain the full chemistry involved in matte smelting. This necessitates adding sulfur to make the product fit the process, and usually none is recovered. From the standpoint of pollution alone, development of a smelting technique for low-sulfur concentrates not requiring the addition of sulfur would be a significant gain.
The Twin Cities Metallurgy Research Center has been engaged in a study to uncover a more direct pyrometallurgical process for winning copper from sulfides. The initial target is concentrate containing primarily chalcocite (Cu2S), and the objective is a smelting technique not requiring the addition of supplemental sulfur. Development work, still in progress, points to a new procedure in which the following sequence of events occur desulfurization, metallization, smelting. It is contemplated that the first two would be conducted sequentially in a 2-stage fluid-bed operation. The third can be carried out in any furnace from which both slag and metal could be tapped. The metal, similar to blister copper, requires refining to remove small percentages of sulfur and iron but this is readily accomplished by well-established industrial procedures.
Desulfurization
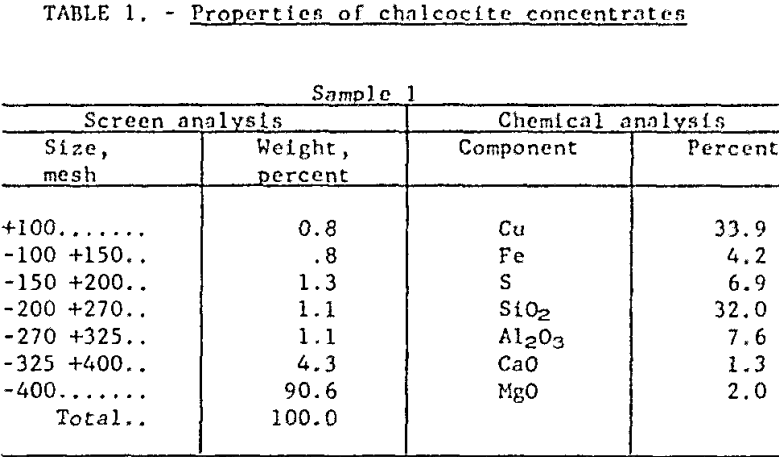
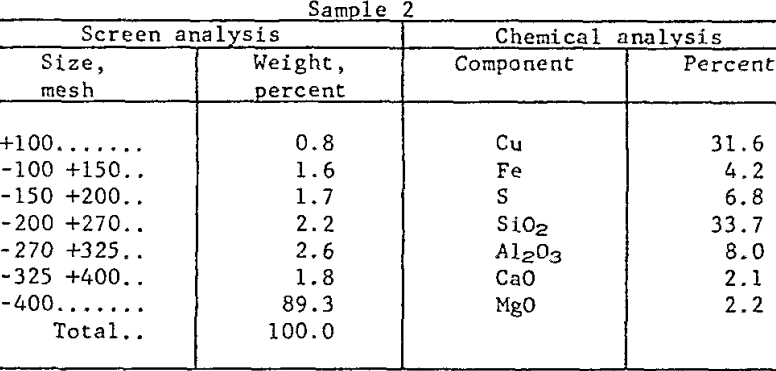
Raw Materials and Procedure: The research has been conducted on concentrates (from a large, integrated mining operation) which were essentially all minus 100-mesh, with 90 percent finer than 400-mesh. Roughly 90 percent of the copper value occurred primarily as chalcocite (Cu2S), with minor quantities of cuprite (Cu2O), covellite (CuS), chalcopyrite (CuFeS2) and bornite (Cu5FeS4). The remaining 10 percent was present as native copper. The major gangue mineral was quartz but there were significant quantities of kaolinite and sericite as well. Table 1 presents chemical and sizing analyses of two samples which are characteristic of present mill practice.
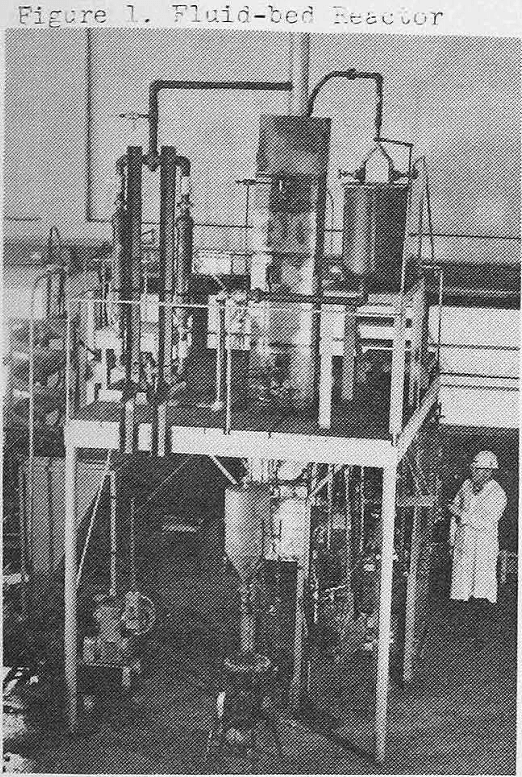
Roasting was carried out in a fluid-bed reactor (figure 1) which is basically a 4-in.-diameter type 316 stainless steel column 9 ft long encompassed by an 18-in. square flue. The flue was low-thermal-conductivity brick supported by a steel frame. The base of the flue was open, and the top cover was connected to an exhaust stack system. The reactor was heated by the combustion of natural gas in four flame nozzles in a ring at the base. Temperature was measured by five thermocouples located along the vertical axis. An automatic temperature controller was connected to the No. 3 thermocouple located 24 inches above the base of the reactor tube. Fluidizing air, after passing through a moisture trap, was regulated by flowmeter and then entered the reactor through a one-inch ceramic ball check valve below the base plate. An overflow discharge pipe, attached to the reactor base plate and extending into the column, established fluid-bed depth which could be varied from 12 to 52 inches. An air-lock table feeder was used for bottom-feeding. Solids to be reacted were entrained with air and carried through the ball check valve and into the fluid column. New feed thus forced an equal volume of roasted materials into the overflow pipe.
Preliminary desulfurization tests with raw concentrates proved unsuccessful because moderate air velocities caused excessive dust losses; low air velocities allowed the particles to adhere to each other, defluidize, and create a fused bed.
In either event desulfurization was very poor. Pelletizing the concentrate solved these problems by permitting greater volumes of fluidizing air.
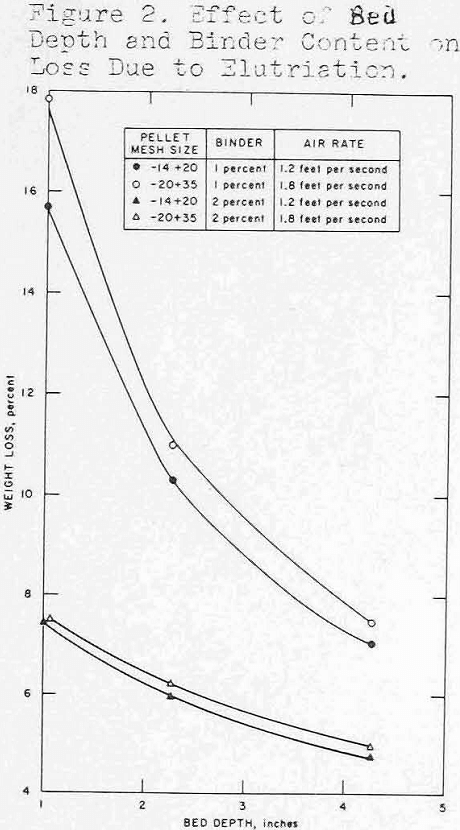
Pellets ranging in size from 35-mesh to 10-mesh were prepared using either bentonite or sulfite liquor as a binder. Because of the small dimensions, the usual criteria for evaluating unfired pellets (e.g., green and dry compression strengths, drop number) were meaningless. An abrasion and degradation test was developed using a 1-¼-in. diameter cold tube as a test vessel in which small quantities of dried mini-pellets were fluidized for 30 minutes at 150 percent bed expansion, and dust losses were determined. The data presented in figure 2 show that deeper beds were a factor in preserving integrity of the pellets.
With the experience gained in some preliminary experiments, it was possible to proceed to round-the-clock operation of the 4-in, reactor on mini-pellets. Measured quantities of chalcocite flotation concentrates, binder and water were blended in a 36-in.-diameter mix-muller and pelietized in a 36-in.-diameter stepped disk. Most of the desulfurization roasts were made on dry, minus 10- plus 20-mesh pellets bonded with one to two percent bentonite.
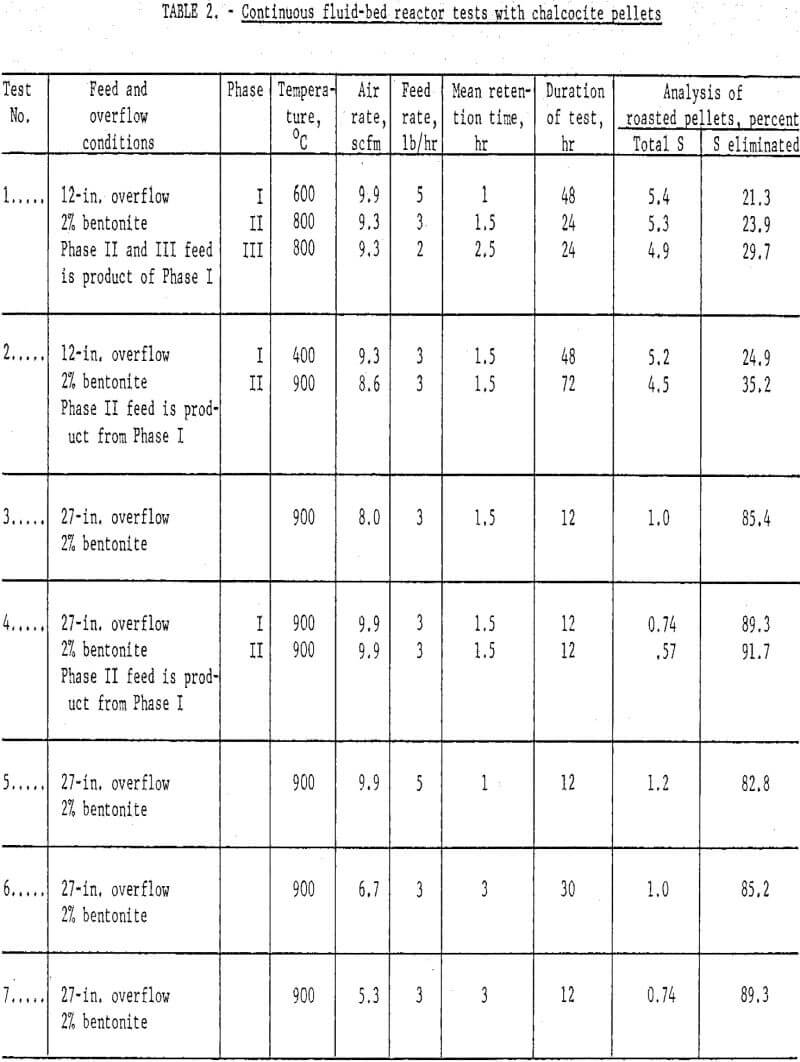
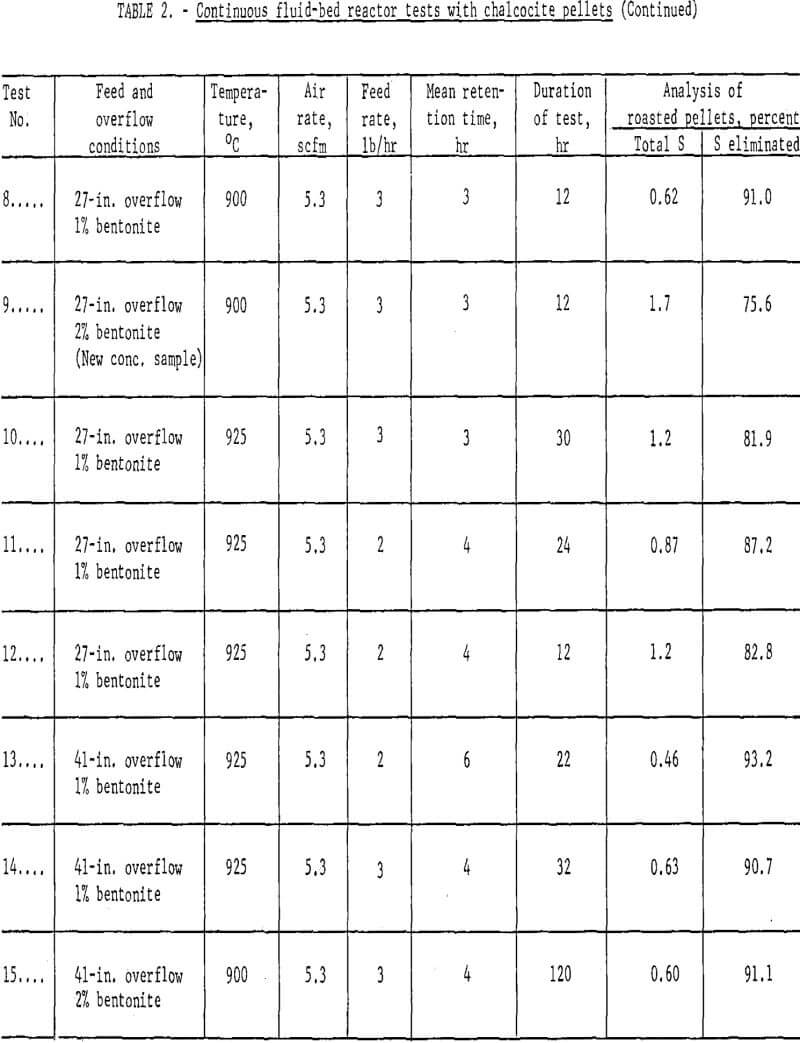
Results: A number of continuous runs were made with variations in temperature, feed rate and bentonite content of the mini-pellets. The campaigns ran from one to five days, but the system reached equilibrium within a few hours, so in the lengthier campaigns, it was possible to complete several individual tests. Some of the data have been gathered in table 2.
An endeavor to achieve more complete elimination of sulfur by 2-stage roasting had the opposite effect. Typical data are the results of tests 1 and 2 (table 2) where the first stage was conducted at either 400° or 600° C and the second at 800 or 900° C. The operation was smooth mechanically, but the sulfur content of the products proved only that normal and basic copper sulfates formed at lower temperatures were extremely difficult to decompose, even at 900° C. Examination of typical products by X-ray disclosed only tenorite (CuO) and dolerophanite (CuO·CuSO4). The microscope revealed small quantities of copper sulfide, either chalcocite (Cu2S) or digenite (Cu1.8S), and cuprite (Cu2O) as well. Chemical analysis showed that soluble sulfates of copper were present in pellets calcined at 900° C. This was contrary to the findings of Wadsworth et al who found only water-insoluble CuO·CuSO4 above 850° C; however, their mean retention time extended beyond 12 hours as compared with usually less than four in the present study. All succeeding campaigns were single-stage tests conducted at 900° – 925° C. Sulfur analyses well under 1.0 percent were achieved.
Concerning the major operating variables, temperature and residence time are most important. Residence time was a function of three factors: feed rate, air flow and bed height. The average retention ranged from one to six hours, but these times were strictly operating estimates. The fluid-bed system is analogous to a stirred-tank reactor; some incoming grains exit almost immediately, and others remain for many hours. Feed rate and bed height exercised the most direct influence on time. Three pounds per hour was established as a good workable feed rate but, in retrospect, it is likely that four or five could have been managed. Similarly, the 12-in. overflow did not use enough of the reactor, but 27-in. and 41-in. overflows were satisfactory.
Air flows determine the bed expansion, so the dilution factor also affects residence time. In the early runs there was a tendency to operate with considerable excess air, but it was gradually brought down to about half the original quantity. A great excess of oxygen to dilute the SO2 evolving from the solids was not essential to insure continued desulfurization; however, a certain air volume was required to dissipate reaction heat and inhibit agglomeration and possible defluidization.
There was a marked quality difference between one percent and two percent bentonite mini-pellets, which was reflected in the dust load. The lost two campaigns of table 2, tests 14 and 15, were quite similar in respects other than bentonite content. Based on product weights the dust in teat 14 comprised 27.5 percent, whereas that of test 15 was only 8.0 percent. The dust could be recycled to the pelletlzer circuit with fresh concentrate. These data point out the essentiality of feeding structurally sound, dry chalcocite pellets. Surviving copper oxide pellets were strong, porous and easily reducible in the following process step.
Metallization of Oxidized Pellets
Raw Materials and Procedure: Following the oxidizing roast the pellets were reduced in the fluid-bed reactor using the bottom-feed procedure described earlier. Several additions were completed beforehand, including a complex gas manifold and a 10,000-ft³ pressure vessel to store reducing gases. Thereafter air, nitrogen or reducing gas could be metered to the reactor. The reductant was 15 percent hydrogen in nitrogen, a commercial heat-treating mixture termed “formed gas” which was neither explosive nor toxic. Both fluidization and reduction were effected by this mixture.
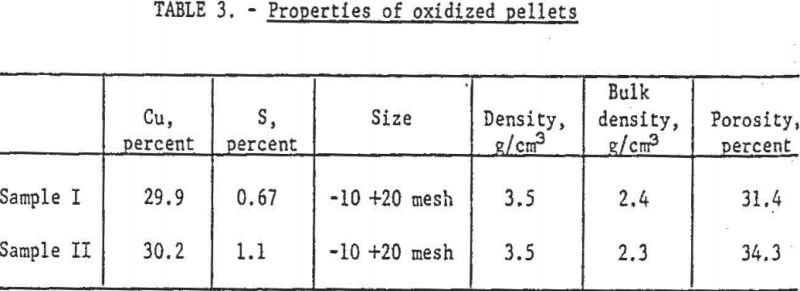
Two composite samples of roasted concentrate pellets, one containing less than 1.0 percent sulfur, the other slightly more, were the feed for metallization. The chemical and physical properties of these pellets appear in table 3.
The reactor was preheated with a bed of the low-sulfur pellets fluidized in air to conserve nitrogen and formed gas. When the reactor attained 750° C at No. 3
thermocouple, a 15-minute flow of nitrogen flushed the system. Reduction was then initiated by switching to formed gas. Concurrently, roasted pellets were bottom fed to the reactor by introducing them into the fluidizing gas stream from the air-lock table feeder. Discharge was through a 36-inch standpipe, the pipe being dumped through a valve every 20 minutes. The hot pellets were protected from oxidation by cooling under nitrogen and sampled hourly. The formed gas at room temperature was metered to the reactor at a rate of 5 scfm for all tests; this is four times the amount theoretically required to metallize the copper contained in the pellets at the highest feed rate used.
Results: Reduction of roasted pellets was a straightforward, trouble-free operation; no operational difficulties were experienced during three tests of 12- to 24-hour duration. Dust (7 percent or less) was recovered by asbestos bags, but was not recycled. Metallization was near 90 percent at 800°, 850° and 900° C, and the average results appear in table 4.
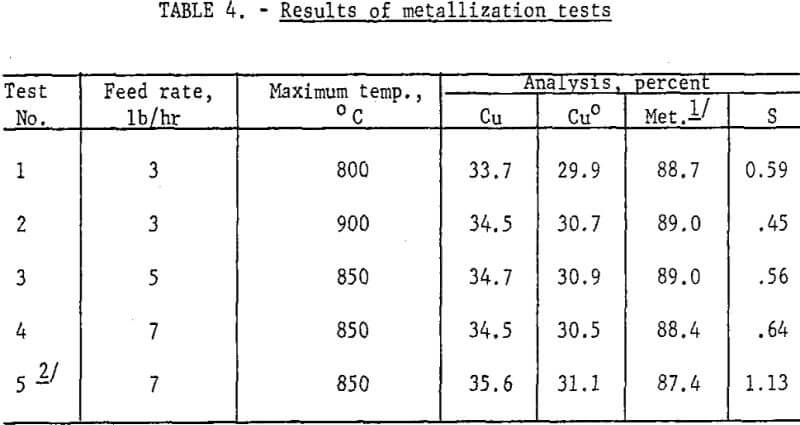
Reduction of CuO to metal is not temperature dependent in the 800° – 900° C range, so precise control was not required. Feed rates were varied from 3 lb/hr to 7 lb/hr and there was a slight, but not necessarily significant, decrease in metallization at the 7 lb/hr level. Retention time was estimated, from an average reactor bed weight of 10.3 lb, to be 3.4, 2.1, and 1.5 hr, respectively, at feed rates of 3, 5, and 7 lb/hr.
Figure 3 is a polished section of a metallized copper pellet. The silicate bond, formed during the oxidizing roast between grains of gangue material (light gray), gives the pellet the necessary strength to withstand the fluid-bed reduction with small dust losses. The metal particles (white) are also bonded to the pellet structure, but since the brittle gangue mineral predominates, the pellets are not malleable. Some of the copper has coalesced (large white grains) and taken on a rounded appearance. This facilitates copper separation from the slag during smelting by increasing the size of the globules.
Copper Smelting
Two factors must be considered in smelting these pellets: (1) They contain up to one percent sulfur which will enter the metal or form a small amount of matte, and (2) not all of the copper oxide is reduced, so small amounts of Cu2O remain in the sample. It is beneficial to process the metallized pellets under slightly reducing conditions, so that what takes place is essentially melting. If the atmosphere is uncontrolled, some copper will reoxidize and then react with the sulfide as follows:
2Cu2O + Cu2S → 6Cu + SO2
2CuO + Cu2S → 4Cu + SO2
Ordinarily, the occurrence of these reactions would be desirable, but in the small laboratory crucibles the persistence of SO2 evolution deterred a sharp slag-copper separation.
Raw Materials and Procedure: Metallized pellets from the fluidized reduction were the feed material for smelting. They analyzed 34.6 percent total Cu, 30.7 percent metallic Cu, 5.2 percent Fe, 0.67 percent S, 10.3 percent Al2O3, and 38.4 percent SiO2. The fluxes used were burnt lime, limestone, and dolomite (table 5).
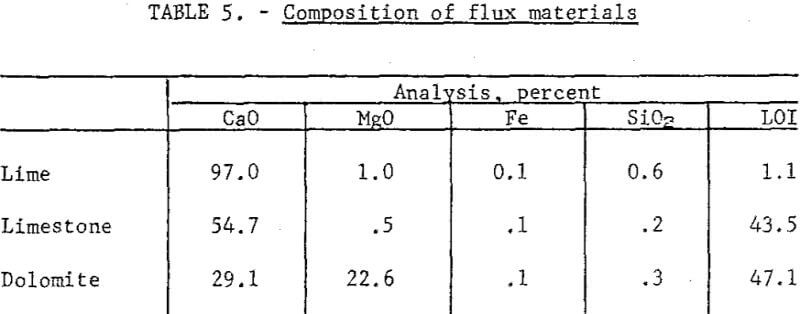
The initial smelting tests were conducted in a muffle furnace, but the results were unsatisfactory; the copper content of the slag generally exceeded several-fold the target level of 1.0 percent copper. Larger batches (500 g) in fireclay crucibles were smelted successfully in a 20-kw induction furnace, the critical factor being the mildly reducing conditions provided by the hot graphite susceptor in the induction furnace. Cooled crucibles containing the smelted charge were weighed and broken, and the contents were separated into slag and copper buttons. Recoveries were calculated from analyses and weights of the feed and slags.
Results: Table 6 presents some of the data from smelting metallized pellets in the induction furnace at 1300° C. Note the very high recovery of copper. The requirements for satisfactory operation included the following: (1) Slag basicity of 0.5 or less, (2) well-metallized pellets, (3) a weakly reducing atmosphere above the bath and (4) formation of a small quantity (less than 7 percent) of matte. The last item was more of an effect than a cause and was a prompt signal that atmospheric conditions had been sufficiently reducing to inhibit oxidation.
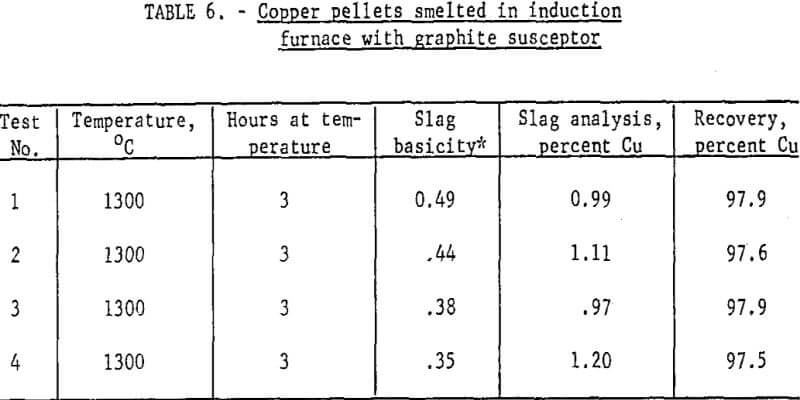
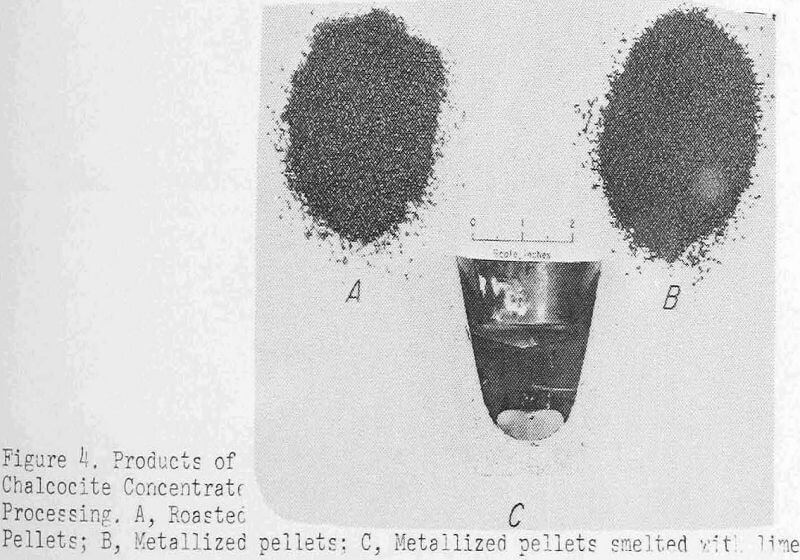
Figure 4 shows some typical products of the processing up to this point. The roasted pellets are the A pile and those in B have been reduced to metal. The black-and-white print does not reveal the change in color but does show the pellets suffer little degradation from the treatment. C is a sectioned crucible after smelting, which illustrates the sharp copper-slag separation possible under favorable conditions.
Copper Refining
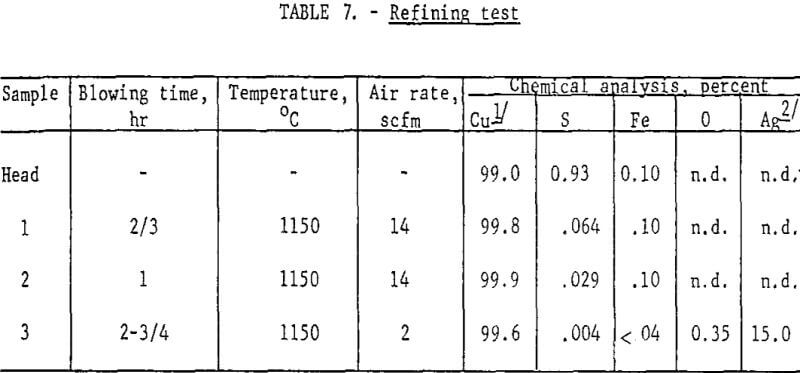
The copper recovered from smelting was contaminated with sulfur and iron and had a composition approximately the same as blister copper (table 7, Head Sample). Fire-refining of such material is a routine commercial operation. A few tests were conducted along these lines to show only that the product would respond to conventional refining procedures. Table 7 summarizes the data.
The head sample was obtained by melting down a 10-lb lot of copper buttons from previous smelting tests and sampling the bath. This was accomplished in an induction-heated silicon carbide crucible. Twenty grams of SiO2 were added, and blowing was initiated by inserting an iron pipe into the molten bath and metering compressed air to the pipe through rubber tubing. The first air rate resulted in an overly vigorous agitation of the bath. After about an hour the liquid level was restored with new feed, another 20 g of SiO2 was added, the air rate was considerably diminished, and blowing continued for 1-½ hours.
The final analysis indicates sulfur and iron had been lowered to satisfactory levels and the metal was ready for poling. Some deoxidation of the metal with hydrogen was carried out but was not carried to completion. However, the few refining tests demonstrated how readily both sulfur and iron could be eliminated.
Conclusion
A new process for recovering copper from chalcocite concentrate includes roasting in air (to about 0.5 percent S) followed by gaseous reduction to metal. These operations, conducted in two states of fluid-bed processing, were successful only when preceded by pelletizing. The metallized material was smelted with 10 percent CaO to yield about 97 percent recovery of Cu in a product similar to blister copper. Blowing the latter with air to simulate refining brought the sulfur and iron contents down to very low values.
Although the research is still underway, the above appears to be a more direct technique for winning copper from chalcocite than the conventional matte-smelting and converting route. Furthermore it is accomplished without the additional sulfur required for matte smelting.