Kaolin is a clay comprised essentially of the mineral kaolinite which has the oxide composition of 46.3% SiO2, 39.8% Al2O3, and 13.9% combined H2O. The structural formula of kaolinite is (OH)8Si4Al4O10. These deposits are sedimentary and contain approximately 90% or more kaolinite along with minor quantities of quartz, mica, and smectite along with trace quantities of ilmenite, anatase, rutile, goethite, zircon, tourmaline, and kyanite. For many uses these accessory and minor minerals must be essentially removed or greatly reduced in amount. The most efficient way to remove these impurities is a wet process, the essential steps of which are shown in Figure 1.
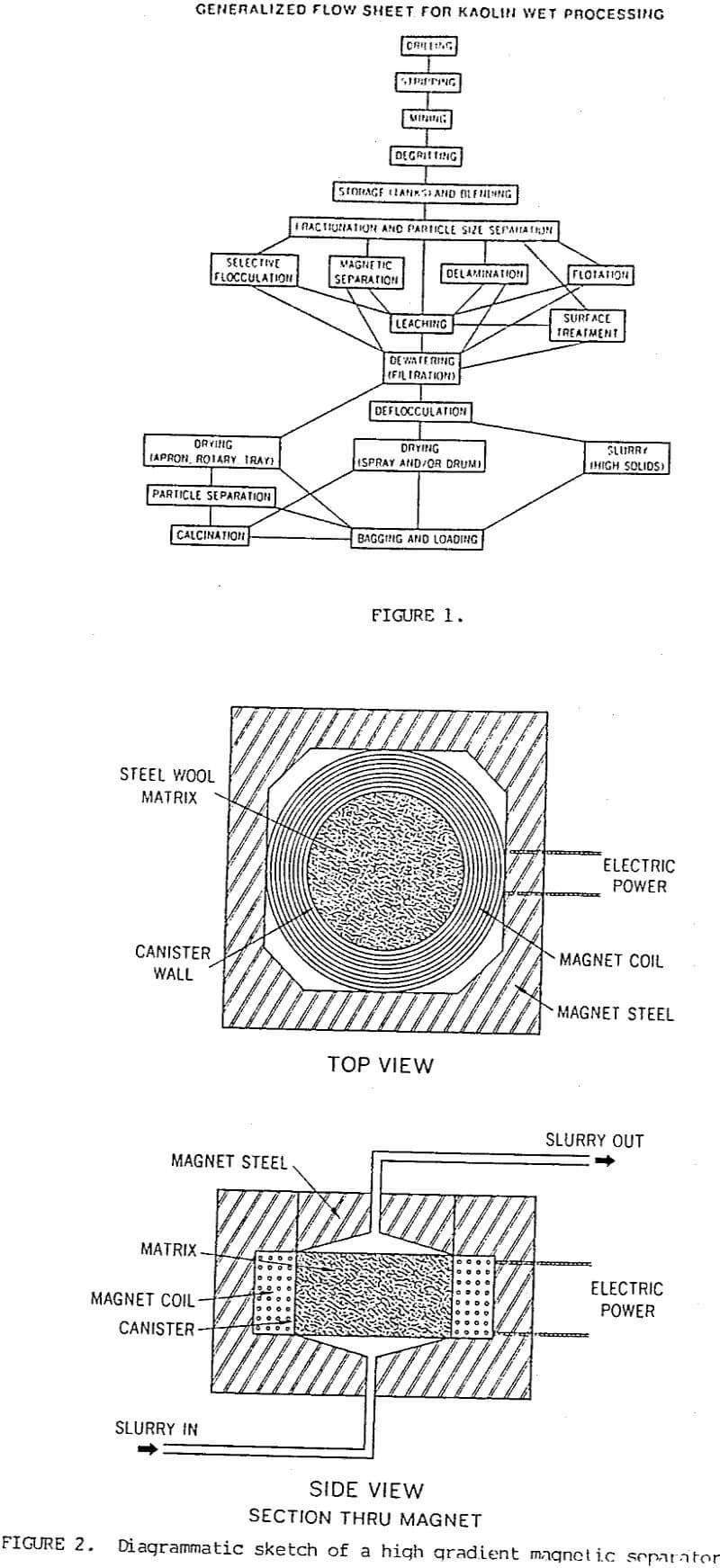
Generally the quartz and mica are present as particles that are coarser than 44 microns (325 mesh) and can be removed by gravity settling and screening. Particle size separations are accomplished by using continuous centrifuges which can separate the kaolin particles into fine and coarse fractions. The particle size of the fine fractions can be controlled between about 70% finer than 2 microns to essentially 100% finer than 2 microns. Any quartz and mica particles that are finer than 44 microns will be contained in the coarse fraction. High intensity magnetic separators are a standard processing technique used in the kaolin industry. This process uses a canister filled with fine stainless steel wool that when magnetized will remove iron and titanium containing minerals from the kaolin slurry as it passes through the canister (Figure 2). A new superconducting magnet has been developed and is in continuous operation in one of the kaolin plants which has reduced power costs and improved the removal of fine paramagnetic mineral particles.
Other processes used to remove Iron and titanium mineral impurities are flotation and selective flocculation. Chemical leaching is a standard beneficiation technique that is used to remove soluble iron. In this process the kaolin slurry is acidified with sulfuric acid to a pH of approximately 3 to solublize the iron. Sodium hydrosulfide, a strong reducing agent, is then added to reduce ferric iron to ferrous iron which then combines with the sulfate iron to form a soluble ferrous sulfate compound which is then removed during the dewatering process. The result of this wet process utilized by the kaolin industry is a very pure kaolinite product. Some of these kaolin products can be tailored to special ceramic applications.
An example of the blending of kaolin with other minerals to make a special ceramic material was in the ceramic support for the automobile catalytic converter. What was needed was a material which had a reasonable firing temperature and a very low thermal expansion. The continual heating and cooling of the ceramic support unit caused severe thermal stresses which caused rapid failure of the monolith unit. From phase diagrams it was determined that the mineral cordierite might have the requisite properties needed for the ceramic support. The firing temperature was about 1300°C and the thermal expansion was 9.8 x 10 -7. Cordierite has the structural formula of Mg2Al4Si5O18 and an oxide composition of 13.7% MgO, 34.9% Al2O3 and 31.4% SiO2. It was then determined that a blend of 37.4% calcined talc (magnesium silicate), 51.73 calcined kaolin (aluminum silicate) and 10.9% alumina (aluminum oxide) would produce a composition that when fired would produce cordierite. This cordierite ceramic support unit for the automobile catalytic converter unit is now used extensively on American and foreign made automobiles.
Some ceramic properties and physical constants of relatively pure kaolin as follows:
Dry shrinkage………………………………………………………………………….4.0 – 7.5%
Green strength (with 50% flint)…………………………………………………300 PSI
Fired shrinkage (1300°C)…………………………………………………………….6 – 15%
Fusion temperature……………………………………………………………………1850°C
Specific gravity………………………………………………………………………………2.60
Hardness (Mohs scale)……………………………………………………………………..2
PCE………………………………………………………………………………………..Cone 33 to 36
The above values are representative of values obtained from relatively pure kaolins. When kaolin is heated the structural water is lost at about 550°C and metakaolin, which is amorphous to x-rays, is formed and at a temperature of about 950°C the metakaolin transforms to gamma alumina and cristobalite. Further heating transforms the silica carrying gamma alumina to mullite and cristobalite.
