Jarosite Production: The roast-leach-electrowin process for zinc production involves roasting zinc sulphide concentrate to produce calcine containing zinc oxide. Zinc concentrate roasted at Risdon typically contains about 11% iron, which is predominantly converted to zinc ferrite (ZnFe2O4) during the roasting operation. When the calcine is leached under weak acid conditions (pH ≤ 2) only the zinc oxide fraction of the calcine is dissolved. The zinc ferrite residue is separated and then leached in a two stage counter-current operation under strong acid conditions (> 50g/L H2SO4; ≥ 85°C). The resultant leach liquor contains up to 30 g/L of iron, which is subsequently precipitated by hydrolysis as ammonium jarosite [NH4Fe3(SO4)2OH6]. The hydrolysis step is carried out at ≥ 95°C, and at about 10g/L H2SO4.
The precipitation of iron as jarosite by hydrolysis generates sulphuric acid and about 40 g/L of H2SO4 must be neutralised in order to achieve near complete iron precipitation. Three neutralising agents are added; ammonia, calcine and a basic zinc sulphate/gypsum precipitate. The ammonia is primarily added as a source of cations (or jarosite precipitation and plays a minor role as a neutralising reagent. In the present Risdon circuit, calcine and the basic zinc sulphate/gypsum precipitate account tor approximately 75% and 25% of the neutralising duty respectively. Under the hydrolysis stage conditions, less than 5% of the zinc ferrite content of calcine is dissolved and 95% is retained in the jarosite waste.
The jarosite pulp is thickened and then neutralised at a pH of approximately 4.0, by the addition of a ferric hydroxide/gypsum precipitate and ammonia. The neutralised jarosite is filtered and extensively washed on the filter before discharge in order to remove entrained zinc plant liquor. The washed jarosite filter cake is repulped in water and transferred to the motor vessel “Anson” for transport to the disposal zone.
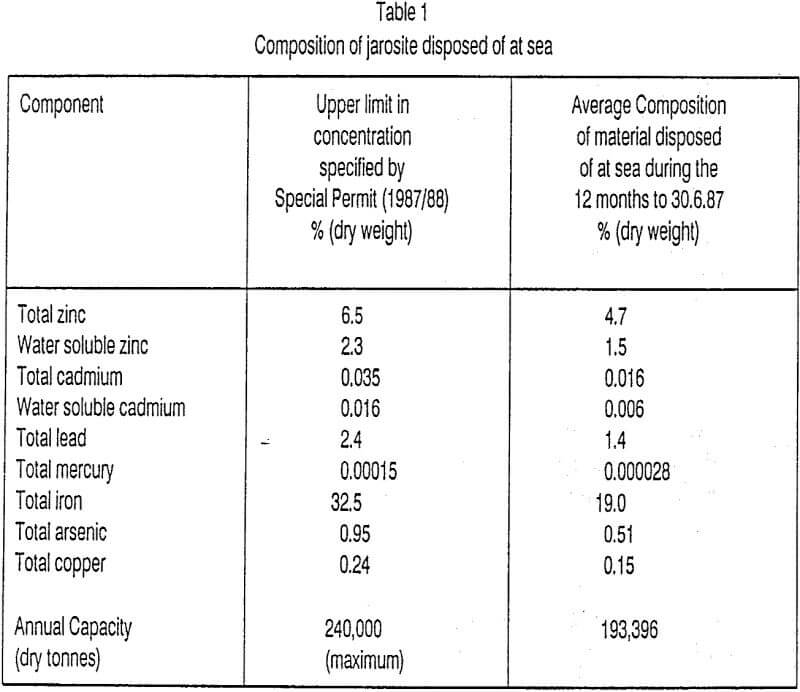
The jarosite disposal rate and composition range for the 12 months to 30.6.87 are shown in Table 1, which also contains details of the upper limits prescribed by the Special Permit which allows EZ to dispose of jarosite waste at sea.
While ammonium jarosite is the most abundant species in the waste, a number of other compounds are present as a consequence of the use of neutralising agents during the hydrolysis step and the subsequent neutralisation of thickened jarosite solids. For instance, the use of calcine as a neutralising agent, is the source of those components of calcine which do not dissolve under weak acid conditions. Thus most of the zinc ferrite and lead compounds present in calcine added to the hydrolysis step report with the jarosite waste. The possibility exists that at least part of the lead may be converted to plumbojarosite (PbFe6(SO4)4(OH)12) during the hydrolysis step.
In addition to the species mentioned above jarosite waste is the main outlet from the Risdon circuit for components such as arsenic, and antimony. Arsenic is the most abundant of such components occurring at levels as high as 0.95%. Arsenic is incorporated into the jarosite waste in a number of ways; by precipitation during the hydrolysis step; by coprecipitation with ferric hydroxide either when the thickened jarosite solids are neutralised or when the aforementioned ferric hydroxide\gypsum precipitate is generated in a separate process step.
Jarosite Disposal
Between December 1973 and January 1988, 5287 shipments of jarosite, involving the disposal of about 2.4 million tonnes (dry weight) were made. For the 12 month period ended June 30, 1987 a total of 193,396 tonnes (dry weight) of jarosite was disposed of, which is 19.4% lower than the permit limit of 240,000 tonnes. Recent operations have typically involved 445 shipments/annum, with the “Anson” unavailable for 29 days/annum.
At the prescribed disposal zone (Figure 1), the jarosite is discharged through doors in the bottom of the holds of the “Anson”. Discharge is rapid and the jarosite forms a plume which gradually settles and disperses. The fate of this plume is the subject of continuing investigation, of which QEM SEM studies are a part.
During the transport of jarosite to the disposal zone there is negligible stratification of the jarosite within the hold. However, on occasions up to 1% of the jarosite waste forms lumps around the discharge doors. These “leathery” lumps may behave differently from the main mass of jarosite, which appears to discharge as a homogeneous entity.
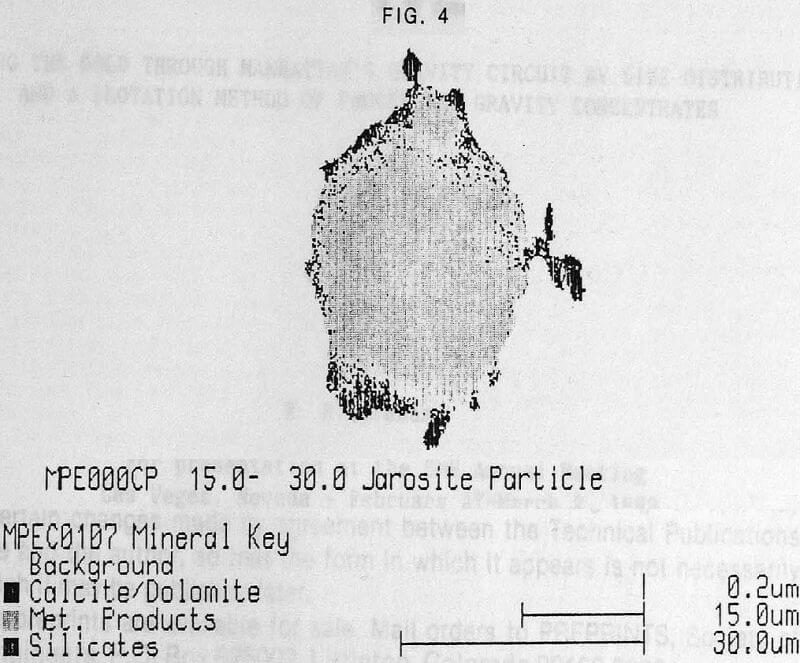
The present studies demonstrate the power of QEM SEM as a technique for investigating the dispersion of particles of metallurgical origin on the ocean floor. The good correspondence between the QEM SEM and wet chemical analysis estimates of the abundance of Fe and Zn is encouraging evidence that QEM SEM provides quantitative information. However, this is neither the real power nor purpose of QEM SEM. It’s advantage is the ability to confirm the chemical identity of compounds, and if necessary to partition the occurrence of an element between materials of marine and metallurgical origin.
A further advantage of QEM SEM is its ability to provide information concerning the physical environment of the metallurgical particles. QEM SEM provides direct evidence indicating the chemical stability of jarosite phases at 2000 meters depth in the marine environment. Evidence to date suggests that the metallurgical particles become associated with the carbonate particles, often becoming occluded within the hollow shells of carbonate material. Assuming that this association occurs naturally, rather than during the sampling and sample preparation procedures, two mechanisms may be postulated. The first is that the jarosite waste settles as a thin layer on the ocean floor and that although this zone is thought to be quiescent, there is sufficient turbulence in the surface layers of the sediments for mixing of the jarosite and carbonate particles to occur. The second and more fascinating is that hollow carbonate particles settling to the ocean floor, actually collect jarosite particles as they pass through the plume of jarosite particles and provide a transport mechanism for jarosite to the ocean floor.

