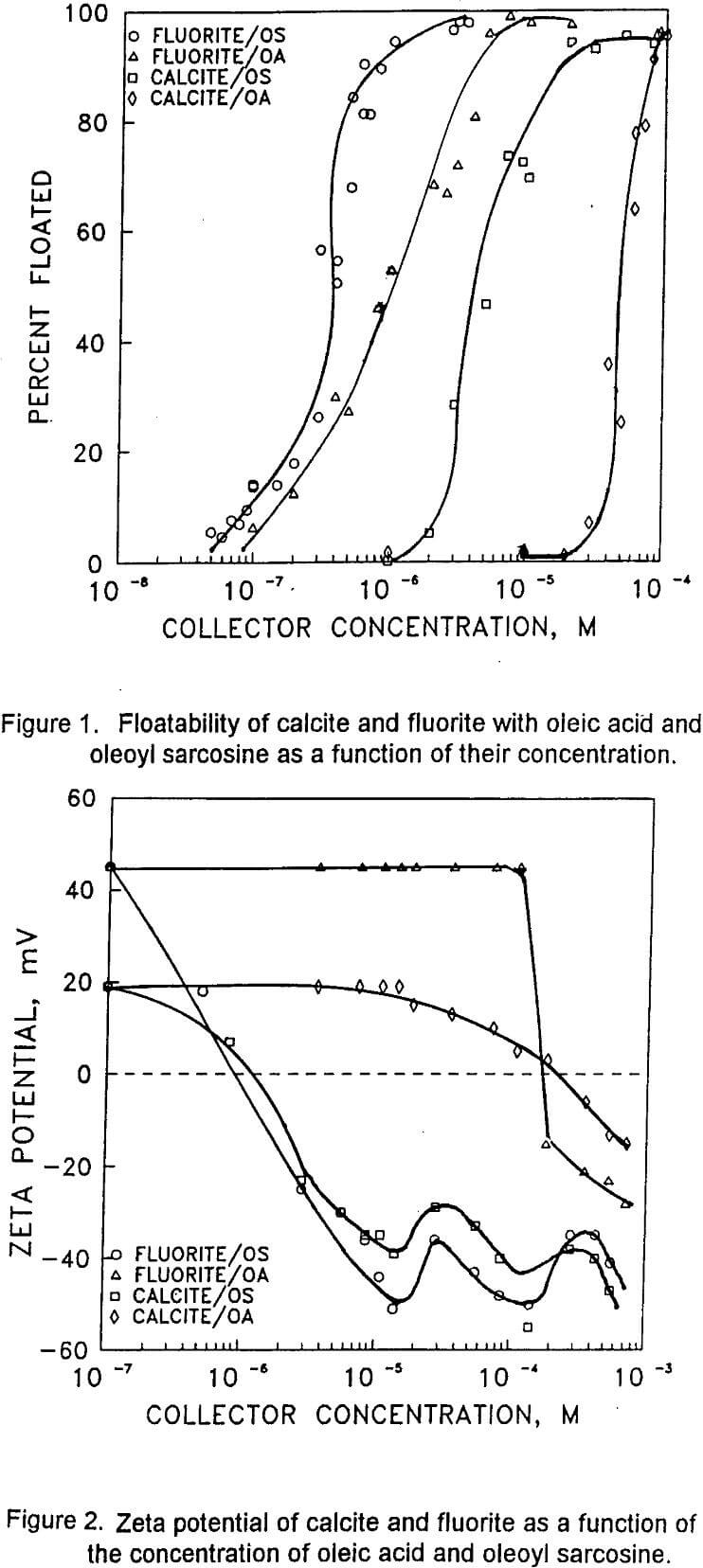
Oleic acid has been almost invariably used in the flotation of siliceous fluorite ores. The performance of oleic acid, however, deteriotates with carbonaceous fluorite ores. Oleoyl sarcosine developed recently has been found to produce results superior over oleic acid with ores containing high calcite contents. While the mechanism of oleate adsorption is usually considered as chemisorption involving calcium oleate formation on both calcite and fluorite, no mechanism has been so far proposed to explain the adsorption mechanism of oleoyl sarcosine.
The separation of sparingly soluble minerals i.e., calcite (CaCO3) from fluorite (CaF2) is largely accomplished by flotation processes. Although both minerals have the same calcium ion in their lattice structure, fatty acids are conveniently used as collectors in this system. Selective adsorption of the collectors at the solid/liquid interface is the primary prerequisite for an effective separation.
Calcite crystals of the highest purity (99.9 %) obtained from Aladag of Turkey were used in flotation, zeta potential and infrared studies. White to light purple fluorite crystals of +3 mm in size were hand picked from the Tavsanli deposit of Turkey. The purity of fluorite was better than 98%.
The floatability of the minerals was determined using a 150-ml column cell (25×220) mm with a fine fritt and magnetic stirrer.
The floatability of fluorite and calcite with oleoylsarcosine (OS) and oleic acid (OA) is a function of surfactant concentration at natural pH values of 7±0.2 and 9.5±0.2, respectively. The ratio of the concentrations at which 100% of the mineral floatable for both fluorite, calcite/OS and fluorite, calcite/OA systems is approximately 20. This indicates that the relative selectivity for both systems is about the same.
Apparently, both fluorite and calcite are positively charged at natural pH but become negatively charged upon increasing the concentration of both reagents. The charge on OS treated fluorite decreases from 45 mV down to -25 mV at a concentration as low as 3×10 -6 M. However, the OA treated fluorite remains positive over a wide concentration range down to 10 -4 M. Similarly, OS treated calcite reverses its charge at 10 -6 M surfactant concentration whereas OA treated calcite undergoes the charge reversal only at 10 -4 M surfactant concentration.
The adsorption bands are not detected up to 50 ppm with both surfactants. The infrared spectra of OA and OS on fluorite and calcite at 250 ppm concentration (corresponding to 7×10 -4 M OA and 9×10 -4 M OS). Evidently, OA adsorbs on fluorite through free -COOH at 1700, 1740 cm-¹ bands (Aplan and Fuerstenau, 1962) and also as (R-COO)2Ca at 1460 cm-¹ due to the the presence of C=O strecthing vibration peaks in its structure. OS/fluorite system at 250 ppm concentration, on the other hand, exhibits a shift in the peak from 1418 to 1380 due to the CH3-N+ bond.
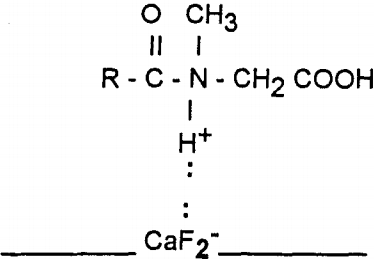
Oleoyl sarcosine floats fluorite and calcite more selectively at approximately five times lower concentrations than oleic acid. The selectivity achieved with oleoyl sarcosine also corroborates with the zeta potential measurements.