Table of Contents
Such matter is presented in this chapter as will give the student some insight into the methods of analysis used in the technical examination of iron and steel. The nature of this work prevents a detailed treatment of the subject—a treatment which may demand an estimation of any one or more of the following substances:—Carbon (free and combined), sulphur, silicon, phosphorus, manganese, titanium, copper, nickel, cobalt, chromium, aluminium, arsenic, antimony, tin, tungsten, vanadium, nitrogen, iron. Generally, the estimations most required are those of carbon, sulphur, silicon, and phosphorus. Of the other elements and compounds mentioned, determinations of one or more are required in the case of special steels. For information regarding these determinations the student is referred to Chemical Analysis and Foundry Chemistry, by Crobaugh ; The. Chemical Analysis of Iron, by Blair ; “Carbon in Steel by Direct Combustion,” by Blount, in The Analyst, Jan. 1902; “Sulphur in Wrought Iron and Steel,” by Auchy, in the Jour. Amer. Chem. Soc., March 1901, and other articles in the same journals. The student who wishes to go further should, if possible, obtain access to the papers and articles of Campbell, Drown, and others, published from time to time in the various chemical and metallurgical journals.
As the student’s time is limited, he may for the present postpone the estimation of silicon and phosphorus, though these are given on account of their importance both to the metallurgist and the foundryman.
In order that the student may obtain a more thorough grasp of the subject, a few notes 0n the composition and properties of the substances considered will not be out of place. Regarding the influence of the various elements on steel, consult The Manufacture and Properties of Structural Steel, by H. H. Campbell.
Carbon exists in iron in three states—graphitic, dissolved, and combined. Besides these, other forms have been identified by the microscope.
Sulphur exists in iron chiefly as the sulphide FeS. which is soluble in molten iron.
Phosphorus exists as phosphide of iron, which is completely soluble in the molten iron.
Silicon forms silicide of iron, which also is soluble in molten iron.
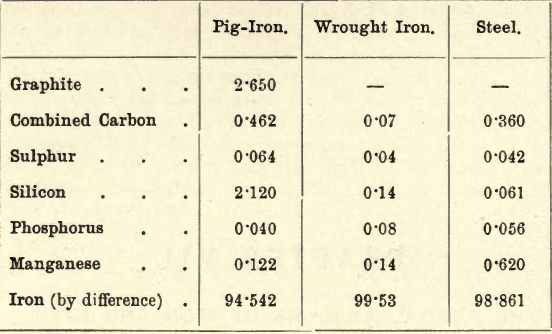
Of these four elements, then, carbon is the only one which can exist in the free state. The variations in the proportions of the various elements present are almost infinite, but the following brief tabulation gives the approximate composition of pig-iron, wrought iron, and steel, though each of these is subject to considerable variation.
The student is required to estimate the following:—
(1) Carbon,
(a) Total.
(b) Graphitic.
(c) Combined.
(2) Sulphur.
(3) Silicon. (If time permits.)
(4) Phosphorus. (If time permits.)
CARBON Total
In this estimation the carbon is converted into CO2 which is absorbed in caustic potash. From the weight of CO2 thus obtained the carbon is calculated.
At first sight it would appear that the simplest procedure would be to ignite the iron or steel borings directly in a current of oxygen and absorb the CO2 thus formed in KHO. Unfortunately this method, so far, has either proved inaccurate, or where complete combustion was obtained, the apparatus necessary to withstand the high temperature or other variations in treatment was not suited to technical work (see articles by Blount in The Analyst). The student will find that the method here given is by no means ideal, from a technical standpoint, on the score of convenience and rapidity, and there seems a probability of it being replaced in the near future by some quicker direct oxidation method.
Method adopted.—On reference to the many works on this subject, a large variety of methods will be found. The method here given will with ordinary care give accurate results. Briefly it is as follows:—
The iron is dissolved in a solution of double chloride of potassium and copper, made acid with HCl. Metallic copper is precipitated and redissolved; the iron is dissolved, the carbon being left in suspension. It is then collected and ignited in the combustion furnace with oxygen, and the CO2 evolved weighed.
Solution of the Iron.—Weigh out 1 gram of pig-iron borings. Transfer to a 300 c.c. beaker. Add 100 c.cs. CuCl2,2KCl,2H2O solution, which is made as follows. Dissolve in water 149.1 parts KCl and 170.3 parts crystallised CuCl2, 2H2O. Evaporate and crystallise out the double chloride. Dissolve 300 gms. of the double salt in distilled water. Filter through ignited asbestos, and preserve in glass stoppered bottles.
To the contents of the beaker add 7 c.cs. HCl to render the solution acid. Stir intermittently till solution of the iron is effected. Place the beaker and contents towards the end of the solution on a water bath at a temperature of about 60° C. The following reactions are taking place—Fe + CuCl2 = FeCl2 + Cu and Cu + CuCl2 = 2CuCl. The KCl simply aids the solution of the precipitated copper. In about 40 minutes from the addition of the double chlorides, solution should be nearly complete and most of the copper dissolved. Wash down the sides of the beaker with a little acidulated double chloride. To the solution add a little ignited asbestos to settle the carbonaceous matter and prevent it clogging the filter (as recommended by Barba).
For filtration, special platinum boats, fitted on the principle of the Gooch crucible, are very convenient. The student may, however, filter off the carbonaceous matter by means of a Gooch crucible aided by artificial suction, the carbonaceous matter being washed in with a jet of water after the liquid has passed through the filter. Carefully wash the carbon on the filter with hot water. Dry the crucible and
contents in the air oven at 100 C. The carbonaceous matter is now ready for ignition.
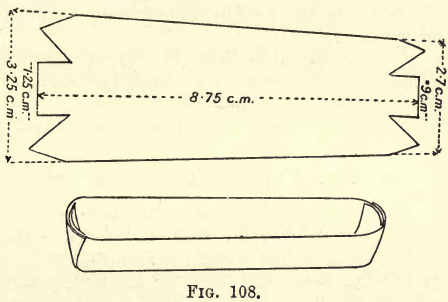
Oxidation of the Carbon.—Prepare a platinum boat by cutting a piece of platinum foil as in fig. 108, folding up the sides and ends to form a trough. Transfer the carbonaceous matter and asbestos from the Gooch to the boat.
The combustion furnace, accessories and fittings must be set in order. The oxygen purification apparatus is again used, but this time is provided with a three-way tube, with taps inserted between the storage and the purifiers. This permits a current of air being drawn through the apparatus. The combustion tube may be of hard Jena glass, porcelain, or platinum. Two U tubes are used between the furnace and the potash bulbs. The limb of the first tube nearest the furnace contains anhydrous CuSO4 the other limb anhydrous CuCl. The second U tube contains dried CaCl2. These two tubes form the ‘purifying train.’ The CuCl absorbs any Cl, and the other substances any H2O. This set will serve for many determinations. The potash bulbs and guard tubes follow, and an aspirator should be handy to draw a current of air through the apparatus when required. The potash bulbs are charged with 8E. KHO, and the guard tube with CaCl2. Test the furnace and bulbs as before described (see Coal and Coke), the tube being charged as in sketch, the boat being for the present kept in the air oven at 100° C.
When all is ready, the burners having been turned out for some time, the boat and contents are inserted. The burners are lighted from the forward end, gradually work backwards, and a slow current of oxygen about two bubbles per second having previously been turned on till the tube is full of oxygen. Regulate the temperature till the boat is at a dull red, and if the solution in the bulbs shows signs of running back to the furnace, increase the current of oxygen to three or four bubbles per second.
From the time of putting in the boat about fifty minutes will suffice for a complete combustion. Turn off the oxygen and pass a current of air for ten minutes.
The potash bulbs and guard tube are now removed and weighed, and the carbon calculated as usual.
(b) Graphitic Carbon —The iron is, by some, dissolved in HCl, by others in HNO3 when the graphitic carbon remains as a residue. For pig-iron either method, with care, gives good results, but for steels containing graphite Blair recommends solution in nitric acid. (For this method consult Blair.)
Weigh out 5 gms. pig-iron borings. Dissolve in 50 c.cs. SE. HCl by aid of gentle heat. Boil for a few minutes. Dilute to 100 c.cs. (nearly). Filter through a Gooch crucible. Wash well with hot water and then with boiling E. KHO. (This dissolves any SiO2.) Wash again with hot water to remove the KHO. Dry the crucible and contents.
Estimate the carbon as before by combustion, and calculate the percentage as usual.
(c) Combined Carbon (by difference).—The total carbon and the graphitic carbon being known, the combined carbon is obtained by subtracting the graphitic from the total carbon.
For direct methods of estimation consult the authorities mentioned.
ESTIMATION OF SULPHUR IN IRON & STEEL
Considerable differences of opinion exist as to the best method of estimating sulphur in iron and steel. The old aqua regia solution and BaCl2 precipitation method is admitted to be very inaccurate; but slow solution in HNO3, with very little or no HCl present, followed by careful precipitation by BaCl2 in presence of a definite excess of HCl and with due care as to time and conditions of precipitation, and precautions against contamination of the precipitate by iron—with these and care good results are obtainable. Blair, on the other hand, recommends solution in HCl, the S being evolved as H2S, which is absorbed in a solution (alkaline) of Pb(NO3)2 forming PbS, which is dissolved in HCl + KClO3, and the S precipitated as BaSO4. For further methods see Blair, Stillman, Auchy, Crobaugh, and Drown. Another method in common use is that of evolution of the S as H2S, followed by absorption in cadmium chloride solution. The precipitated cadmium sulphide is dissolved in HCl and the S estimated by titration with an iodine solution, or more common still, the H2S is absorbed in Br. water and then precipitated as BaSO4 or is absorbed in NaOH and titrated with iodine; this latter being the favourite method. (See Blair.) The following method is here given:—
Oxidation by HNO3 (the so-called Aqua Regia method).—Weigh out 5 gms. borings and transfer to a deep 200 c.c. beaker. Carefully add about 40 c.cs. 16E. HNO3, in lots of about 10 c.cs. at a time, covering the beaker with a large watch glass and taking care that the action is not too violent. When the action apparently ceases, note if all the particles are dissolved (except any carbon). If not, heat on the sand bath, and add 3 or 4 drops of 16E. HCl, and warm till dissolved.
When solution is complete, add a little Na2CO3 to convert any H2SO4 into Na2SO4, which is non-volatile on evaporation.
Remove from the sand bath, and add 5 c.cs. of strong HCl in excess of that necessary to just dissolve any iron compounds precipitated by the Na2CO3. Filter off the SiO2, and C. Wash well. Evaporate to dryness to render SiO2 insoluble. Take up with HCl and evaporate till Fe2Cl6 begins to crystallise out. Then add 5 c.cs. HCl. and filter if any residue is present. (If none is present, no SiO2 was in solution, and the evaporation could have been omitted.) Filter and wash carefully the precipitate in the Gooch, bringing the liquid and washings up to about 100 c.cs.
Heat to boiling. Add 10 c.cs. saturated solution of BaCl2. Boil for 30 minutes. Allow to stand over-night. Filter through a Gooch. Wash with a little E. HCl. and then with water. Dry, ignite, and weigh as usual the BaSO4, which should be white, and not contaminated with iron salts.
Calculate the percentage of S in the usual way. As some of the reagents used may contain sulphur, a blank must be run, using the same quantities as in the actual analysis, and any sulphur found deducted from the previous result.
ESTIMATION OF SILICON
The method here given is that of Drown, and is both rapid and exact. The iron is dissolved in HNO3, followed by H2SO4, with evaporation to dryness. This is followed by solution, leaving the silicon in the residue as SiO2.
Details.—Weigh out 2 gms borings, and transfer to a platinum or porcelain dish. Add 30 c.cs. 8E. HNO3 When the action apparently ceases, add 20 c.cs. 18E. H2SO4, and evaporate. (Blair recommends a gentle blast of hot air playing on the surface of the liquid. The air is heated by passing it through a small spiral of copper pipe heated over a bunsen. Evaporation is thus hastened and spirting prevented.) Continue the evaporation until copious fumes of SO3 come off. Cool, and cautiously dilute with distilled water to 130 c.cs. Heat till all the FeSO4 is dissolved. Filter, and wash first with a little E. HCl, and then with hot water. This filtration is best performed with a 7 cm. ashless filter paper (check the ash by igniting two or three of the papers). Dry; transfer to a platinum crucible; ignite as usual and weigh. To the crucible add 5 c.cs. strong H2SO4 and 5 c.cs. strong HF. Carefully evaporate to dryness, using a hot blast of air to hasten the evaporation. Ignite, and weigh again. Provided the H2SO4 and HF are pure, the difference in weight represents SiO2. Check the H2SO4 and HF (particularly the latter) by evaporating a blank. Any residue found must be allowed for.
ESTIMATION OF PHOSPHORUS
Here, again, numerous methods are given by different authorities, the majority of them yielding accurate results when carefully followed out. The two methods most suited to technical analysis are the volumetric reduction method prepared by the sub-committee (Messrs Barba, Blair, Drown, Dudley, and Shimer) of the International Steel Standards Committee, U.S.A., and the modified reduction method as given by Messrs Dudley and Pease, Jour. Anal. Appl. Chem., vii. 108. The former method is fully discussed in Blair’s Analysis of Iron; the latter method is given here.
The iron is dissolved, and the P precipitated as phospho-molybdate of ammonium. This is dissolved, and by the action of Zn and H2SO4 the MoO3 is reduced, and the reduced liquid is then titrated with K2Mn2O8 (standard solution), and from the number of c.cs. used the P contents may be calculated.
Details. —Where much work is to be done, a shaking apparatus is necessary (see Chemical Supply Catalogues). The student, however, may perform the necessary shaking by hand. Before proceeding to the analysis, the reducing apparatus (a modification of the Jones reductor) must be prepared (see fig. 109).
At a is a finely perforated disc of stout platinum foil. Between a and c is about ¾ inch of clean white sand, c is another perforated platinum disc.
Above this disc the tube is filled with fine granulated amalgamated zinc, prepared thus:—Dissolve 5 gms. Hg in 25 c.cs. strong HNO3, diluting with water and making the solution up to 1 litre. In this solution
pour half a kilo of granulated zinc which passes a 20 but not a 30 sieve. Shake for one or two minutes. Pour off the solution. Wash and dry the zinc, which is now amalgamated. The funnel and flask are fitted to the apparatus as shown.
Prepare the following Reagents :—
(a) The Strong Oxidising Solution of K2Mn2O8. 12 gms. pure K2Mn2O8 in 1 litre water. Filter and bottle.
(b) The Molybdate Solution.—Dissolve 50 gms. MoO3 in 200 c.cs, NH4HO (S.G. .96). Filter, and to the filtrate add 500 c.cs. HNO3 (S.G. 1.2). Let stand at least 24 hours before using.
(c) The Acid Amnwnium Sulphate Solution.—To 500 c.cs. distilled water add 27.5 c.cs. NH4HO (S.G. 0.96), and then 24 c.cs. pure H2SO4 (S.G. 1.84), and dilute to 1000 c.cs.
(d) The Standard K2Mn2O8 Solution.—Dissolve 2 gms. crystallised K2Mn2O8 in 1000 c.cs distilled water. Standardize the solution as follows : Weigh out 3 lots of from .1 to .3 gm. each of thoroughly cleaned iron wire, the iron contents of which are known. Transfer to 100 c.c. Erlenmeyer flask, and add to each 40 c.cs. 8E. H2SO4. When dissolved, boil 5 minutes ; dilute to 150 c.cs., and pass through the reductor and wash, bringing the volume up to 200 c.cs., as directed in the analysis. Titrate each lot with tlie K2Mn2O8. The results should agree for metallic iron to 1/100 milligram. Make the required allowance for the impurities in the wire taken. Suppose 1 c.c. K2Mn2O8 = .0034923 gm, Fe, then multiply this value in Fe by the ratio of MoO3 to Fe, namely, .9076, and the product by ratio of the P
present to the MoO3 namely, .019, we have
1 c.c. K2Mn2O8 = .0000602 gm. P
Analysis
Weigh out 1 gm. borings. Transfer to a 200 c.c. Erlenmeyer flask. Add 70 c.cs. 5E. HNO3. When solution is complete, boil a minute, and add 10 c.cs. of the ‘oxidising’ solution of K2Mn2O8. Roil till the pink colour disappears and MnO2 separates out. Remove, and add gradually with stirring crystals of pure (phosphorus free) FeSO4 till the contents clear up. Heat the solution to 80° C. (if As is present, to 35° C.). Add 75 c.cs. of the molybdate solution at a temperature of 27° C. Close the flask with a rubber stopper and shake for 5 minutes. Let stand for 5 minutes. Then filter through a 9 cm, filter, and wash with the acid amm. sulphate solution till a few drops of the washings give no colour with ammonium sulphide.
Dissolve the precipitate on the paper with 5 c.cs. NH4HO (S.G. 0.90) and 25 c.cs. water, letting the solution run back into the original flask, thus dissolving any precipitate adhering to its sides. Wash till filtrate and washings amount to 150 c.cs. Add 10 c.cs. strong H2SO4 (S.G. 1.84), and dilute to 200 c.cs. The solution is now ready for reduction.
Pour 100 c.cs. warm ~E/2 H2SO4 into the funnel. Connect the flask to the filter pump and open the clamp, so that the solution nearly, but not quite, flows out of the funnel. Then to the funnel add the following blank—5 c.cs. NH4HO (S.G. 0.90), 10 c.cs. H2SO4 (S.G. 1.84), and 50 c.cs. water, mixed together. Again open the clamp, so as to nearly run this mixture out of the funnel. Now add 200 c.cs, E/2 H2SO4 to the funnel, and nearly run through.
Disconnect the flask, first closing the stopcock oil the funnel. Titrate the contents of the flask with K2Mn2O8. Generally about 0.1 c.cs. permanganate are thus consumed, and this quantity must be deducted from future readings.
Now transfer the solution to be reduced to the funnel. Attach a clean flask. Connect and start the filter pump. Open the stopcock and clamp, so as to nearly run through the solution. Wash out the flask that contained the solution with 100 c.cs. E/2 H2SO4. Add this to the funnel, and treat as before.
Finally, add and nearly run through another 100 c.cs. of the acid.
The reduced solution in the filter flask should now be bright green.
Remove as before and titrate with the permanganate solution. The green changes to pinkish brown, then pinkish yellow, then colourless, and finally a permanent pink is obtained (after standing one minute). From the reading obtained deduct the blank reading, and calculate the percentage of P present from the data given above.
Instead of this volumetric method, some chemists prefer to weigh directly the yellow phospho-molybdate precipitate. For details see Blair’s Analysis of Iron, p. 108.
Note.—The student should, wherever possible, take advantage of references to special authorities. By this time he should be capable of consulting, comparing, and, to some extent, judiciously using such materials. No one text-book can give an anyway comprehensive treatment of ‘ Iron and Steels,’ or, for that matter, of any one of the subjects treated of in this section; therefore such references as are given, together with current literature, must be carefully perused by the analyst who wishes to excel in technical work. The colorimetric determination of combined carbon by Eggertz’s Method has been given ; manganese may be determined somewhat similarly by Peter’s Colorimetric Method, or by the Acetate Method (see Blair, etc.).
