Carboxylate can interact with metal cations in four different modes, namely ionic, unidentate, bidentate, and bridging as depicted in Figure 9. The ionic type of interactions are usually observed in the alkali metal salts of carboxylic acids. In this case, the carboxylate ion has a symmetrical structure and the two oxygen atoms in the carboxylate group are associated equally with the metal cation. The characteristic bands for the carboxylate are in the range of 1510-1650 cm-¹ for the asymmetrical vibration and 1280-1400 cm-¹ for the symmetrical vibration.21,28 In the case of sodium oleate, it was found that the symmetrical and asymmetrical bands were located at about 1440 and 1560 cm-¹ from ex-situ diffuse reflection spectra (not presented).
For the unidentate carboxylate group, since only one oxygen atom is coordinated with the metal cation, the symmetry of the functional group is lower when compared to that of the carboxylate group in the ionic form. An increase in the asymmetrical stretching vibration and a decrease in the symmetrical stretching vibration are observed, resulting in a larger separation of the asymmetric and symmetric stretching frequencies (Δv=va-vb). Separation from about 200 cm-1 to about 300 cm-¹ has been reported for unidentate metal acetates. The asymmetric and symmetric vibrational frequencies in the unidentate carboxylate are closer to the vibrations of C=0 (∼1700 cm-¹) and C-O (∼1400 cm-¹) in the carboxylic acid form.
The carboxylate group in the bidentate mode has the same group symmetry as in the free ionic state. Therefore a decrease in the asymmetrical vibration frequency and an increase in the symmetrical vibration frequency (smaller separation ) are expected relative to those of carboxylate in the unidentate coordination state. An asymmetrical vibration of 1550 cm-¹ and symmetrical vibration at 1456 cm-¹ (Δv=va-vb=94 cm-¹) has been reported for bidentate carboxylate, Zn(O2CCH3)2 2H2O.
The bridging coordination of carboxylate bears similarity to the unidentate coordination because in the bridging coordination only one oxygen coordinates with one particular cation. But the group symmetry in the bridging coordination state is similar to that in the bidentate coordination. Therefore, the IR spectrum of the carboxylate in bridging coordination state is expected to be similar to that of the carboxylate in bidentate carboxylate (in term of band positions and separation). It has been reported that the bidentate coordination of carboxylate causes the OCO angle to decrease in comparison to the free ionic form and the bridging coordination. Therefore, relatively higher band positions (in wavenumbers) may be expected for the carboxylate vibration in the bridging coordination mode. The following wavenumber position for the asymmetric vibration of carboxylate is expected, Vunidt> Vbrid>Vbidt.
Keeping the above discussion in mind, it can be stated that although the different coordination states of the carboxylate correspond to different vibrational band positions, no single coordination state gives rise to the splitting of the asymmetrical vibration of the carboxylate in calcium carboxylates. The observed splitting of the asymmetrical vibration may come from two differently coordinated carboxylate groups in the calcium-carboxylate complex. This observed splitting in the asymmetrical vibration is specifically associated with most calcium carboxylate salts and is not observed for magnesium, barium, and other carboxylate alkali earth metal salts. Therefore, the splitting in the asymmetrical vibration appears to be related to the unique coordination characteristics of calcium ion in aqueous systems.
Coordination of Calcium Ions with Carboxylates It has been shown that calcium ions overwhelmingly prefer to bind oxygen atoms rather than other atoms. Water and carboxylate groups are the most common and stable ligands. Six, seven and eight coordinate structures are preferred by calcium ions with water and carboxylate ligands. It is common for calcium ions to coordinate with both water and carboxylate ligands. In the survey by Katz et al. it was found that when calcium ions bind carboxylate ligands in a structure of 6-fold coordination or less it appears that the carboxylates associate with the calcium cation in a unidentate fashion only. When calcium ions bind ligands in 7- and 8-fold coordination, both unidentate and bidentate coordination between calcium cation and carboxylate ligands are possible. It was also found in the same work by Katz et al. that when calcium ions coordinate with water in an uneven number i.e. five and seven ( three is an exception) the coordination structures are asymmetrical and in some circumstances, the coordination structure of the calcium ion is chosen stereochemically. It has also been reported that the interactions between carboxylate groups and calcium ions have the following characteristics. (1) calcium ions generally lie near the planes of the carboxylate groups, (2) bidentate interaction confines the calcium ions predominantly around the bisector of the carboxylate group, and (3) in the unidentate coordination, calcium ions can be found on either side with one of the oxygen atoms. These characteristics are very useful in describing carboxylate interactions with calcium ions.
With respect to the singlet/doublet issue associated with adsorbed carboxylates at semisoluble calcium-bearing mineral surfaces, three IR peaks have been identified for the asymmetric carboxylate vibration, i.e. the peaks at 1540, 1550, 1570 cm-¹. According to the above discussion, these peaks can be tentatively assigned to carboxylate binding calcium cations in the bidentate, bridging, and unidentate fashion, respectively. More work on crystal structure of long chain calcium carboxylate salts needs to be carried out to obtain experimental evidence for these assignments.
When carboxylate is adsorbed from dilute solution, before monolayer coverage is complete and under conditions where no calcium dicarboxylate salt precipitates (the case for low carboxylate concentrations, referring to the results in Figure 1), bridging of the carboxylate may be preferred because the distance between the two oxygen atoms is about 3.5 A. and the distance between two closest calcium ions at the fluorite surface is about 3.86 A. Taking the bond length between calcium and oxygen atoms of the carboxylate into consideration, the distance between the atoms in the carboxylate group match the nearest calcium distance at a fluorite surface very well. Also considering the unit cell parameter of ao=5.462 A for the fluorite crystal, it has been calculated that calcium atom surface density at the fluorite (111) surface is 12.9 (μmole/m². The close-to monolayer adsorption density plateau of oleate spontaneously adsorbed at fluorite surface has a value of 6.5-6.8 μm/m2. A calcium to carboxylate ratio of close to 2 to 1 for the fluorite (111) surface is obtained when covered by a monolayer of carboxylate. These calculations also favor the bridging interaction between carboxylate groups and calcium ions at a fluorite surface especially at the (111) surface which is the natural cleavage plane. As discussed previously this bridging of carboxylate gives rise to the asymmetric vibration at about 1550 cm-¹ which is observed in the oleate/fluorite system for adsorption from low concentration solution in this work and by other researchers. This bridging of carboxylate is limited to a maximum coverage of a monolayer (due to the availability of surface reactive sites) and is considered to be the chemisorbed carboxylate. The great stability of the adsorbed carboxylates at fluorite surfaces seems to support this position.
Transferred LB stearic acid, representing a well organized monolayer film, reacts at a fluorite surface as evidenced by the absence of the C=O at about 1710 cm-¹ and the appearance of the asymmetrical carboxylate vibration at 1550 cm-¹. This result again strongly supports the chemisorption characteristic of a monolayer of carboxylate spontaneously adsorbed from dilute solution at the fluorite surface.
Calcium carboxylate precipitated from bulk solution, and carboxylate adsorbed under conditions where calcium carboxylate precipitation occurs, have been characterized by a splitting of the asymmetric vibration of the carboxylate group. This splitting seems not to be due to the order of the carboxylate organization as proposed by Mielczarski et al. but appears to arise from the coexistence of molecules binding the calcium cation in different fashions. The phenomenon seems to be calcium specific, and is not the case for magnesium, cadmium, and most other divalent metal carboxylates. It has been reported that the average coordination numbers for magnesium and calcium with water are six and seven respectively. As calculated from a molecular orbital study, for 7-fold coordination calcium complexes the complex structures are asymmetrical. This asymmetrical structure may be better fitted by accommodating some carboxylate molecules in a unidentate fashion and others in a bidentate coordination as opposed to a symmetrical structure. This coordination of calcium may be the reason for the splitting of the asymmetrical carboxylate vibration. Crystal structure determination of Sn(O2CCH3)4 shows that the tin is in 7-fold coordination with the oxygen atoms of two bidentate and three unidentate carboxylate atoms. The corresponding IR spectrum reveals an absorption doublet at about 1570 and 1636 cm-¹ for the asymmetric carboxylate stretching vibration and 1310 and 1430 cm-¹ for the symmetric vibration. A 7- fold coordination structure has also been observed for the α-calcium formate in which some of the carboxylates coordinate with calcium in a bidentate way and other carboxylates in a unidentate way.
A similarly structured complex may exist between calcium and carboxylate as long as it is stereochemically possible.
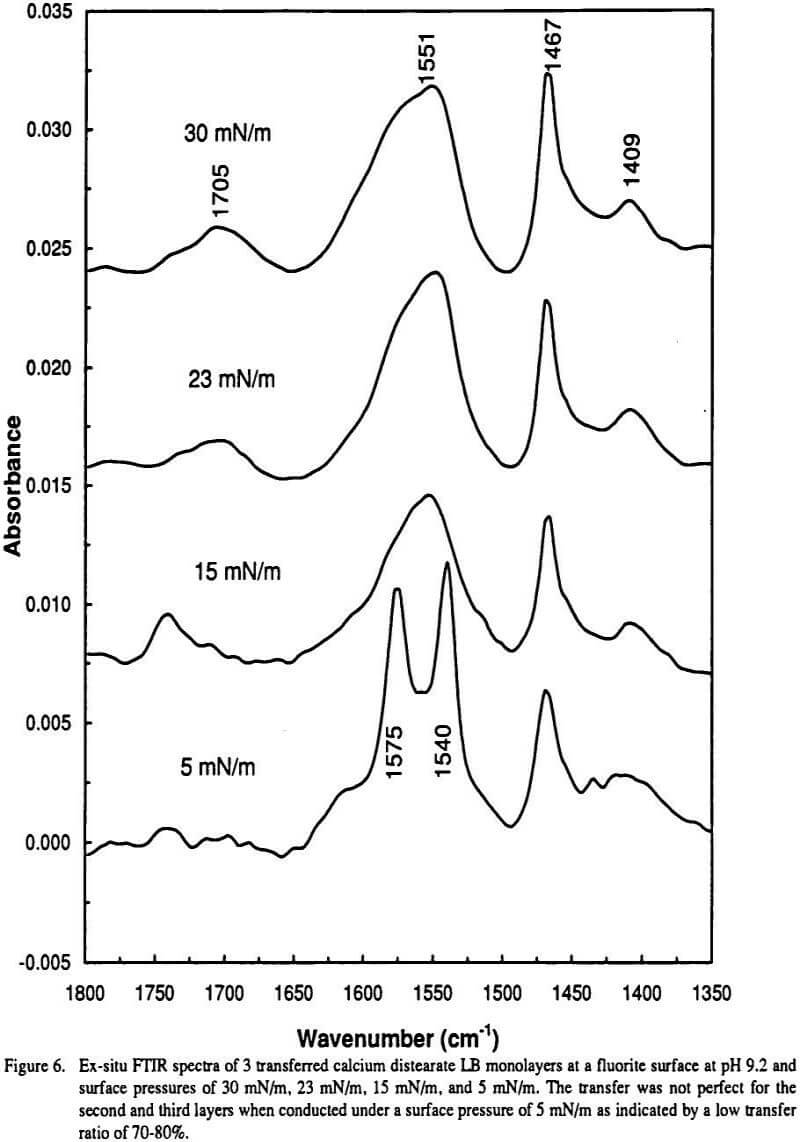
These 7-fold coordinated calcium carboxylate complexes may contain water inside the complex and have a three dimensional structure in space. If such space is not allowed or under some circumstances, where the 7-fold coordination is forbidden, the splitting of the asymmetrical vibration will disappear as is the case for LB transferred calcium distearate at a fluorite surface where the carboxylate groups are all confined in one plane (the air/solid interface).
Finally the 7-fold coordination structure may be destroyed by high pressure treatment, as was observed in the preparation of a KBr pellet (Gong, et al.), and by vacuum treatment under elevated temperature (Mielczarski, et al.). Under these circumstances the doublet is reduced to a singlet Also, when the surface confinement is not strong enough (for example, transfer is conducted under low surface pressure or imperfect layers are transferred) the carboxylate has spatial freedom to form the thermodynamically favored 7-fold coordination as is the case for transferred calcium distearatc at a hydrophobic surface or under low pressure at a hydrophilic surface ( Figure 6).
![]()

