Table of Contents
Mix about 1 or 2 grams of potassium iodide with about twice its weight of manganese dioxide, and transfer the mixture to a flask fixed on a retort stand, then add a few c.c. of strong sulphuric acid (do not fit a cork into the flask); violet fumes soon appear, and condense in the neck of the flask, forming bright metallic-looking plates. Collect a few of these crystals.
2KI + MnO2 + 2H2SO4 = K2SO4 + MnSO4 + 2H2O + I2
Iodine Gas Laboratory Experiment I
Take a crystal of iodine and shake it up with water in a test-tube; the water is slightly tinged yellow, but very little is apparently dissolved. To this solution add a little starch paste; a blue colour is at once produced. Heat this solution and the colour disappears, but reappears again on cooling. This test affords an easy means of detecting the presence of iodine.
Laboratory Experiment with Iodine Gas II
Dissolve a crystal of potassium iodide in water in a test-tube, add a few drops of starch paste ; no blue coloration is produced. Add a drop or two of chlorine water; the blue colour instantly appears. Add more chlorine water, and the solution becomes colourless, owing to the formation of a chloride of iodine which has no action on starch.
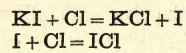
Laboratory Iodine Gas Experiment III
Mix solutions of potassium iodide and bromide together in a test-tube, add carefully a little chlorine water; the liquid becomes a yellowish-red colour. Now add a little carbon disulphide and shake the tube; the iodine is dissolved in the carbon disulphide, giving it a violet colour. Draw off the solution from the carbon disulphide by means of a pipette, and to this solution add more chlorine water and carbon disulphide, and shake; the bromine is now dissolved, imparting to the carbon disulphide an orange colour. Iodine and bromine can thus be detected in the same solution.
INSTRUCTIONS TO THE STUDENT
The student has now obtained a fair knowledge of simple glass-working and of the preparation and properties of the more common gases. In the following sections he proceeds to examine solids and liquids (generally solids which are put into solution). First he examines an unknown substance by ‘dry tests’ for ‘ bases ’ and ‘ acids ’; the results so obtained are then confirmed by systematic ‘ wet tests.’
The order of work laid down is to be followed with most careful and patient attention to all details; the student has then done his part, but unless this is supplemented by equal care on the part of the demonstrator, the best results cannot be expected. The demonstrator, besides supervising the actual testing, must prepare a carefully graded series of substances leading from simple salts containing one ‘base’ and one ‘ acid ’ to complex mixtures containing four or five ‘bases’ and several ‘ acids ’ (including insolubles). The substances must all be carefully selected with the definite object of teaching the student some important point in every mixture he analyses. Indiscriminate preparation of mixtures leads to waste of time and bad work.
In his first tests the student may be given salts of known composition, and his work is then checked by unknown salts from the demonstrator’s set. For instance, on the next page he may take ZnCl2, SnCl2, Pb, Bi2S3, and so on for practice, and when fairly confident, his proficiency or otherwise is checked on ‘unknown’ salts given by the demonstrator.
The demonstrator’s ‘Record Book’ should show full details of the substances given to each student, the results obtained, the time taken with the analysis, and general remarks where necessary. A somewhat similar record must be kept of the work done in Quantitative Analysis and Assaying.
