Table of Contents
The pressure oxidation process is especially suitable for the sulfide type of refractory ores. It includes two stages of operation. First, the refractory ore is subjected to an autoclave oxidation. At elevated temperatures usually above 180°C and in acidic or alkaline medium and pressurized oxygen, sulfide minerals are oxidized for a period of 1-2 hours. The slurry is then discharged from the autoclave and neutralized with lime, the pH of the solution being adjusted to 10-11.5. Second, this slurry is subjected to conventional cyanidation. The overall operation needs 24-48 hours, depending on type of the ore. Obviously, the process is poor in efficiency and is costly, and also suffers from the fact that cyanide is used.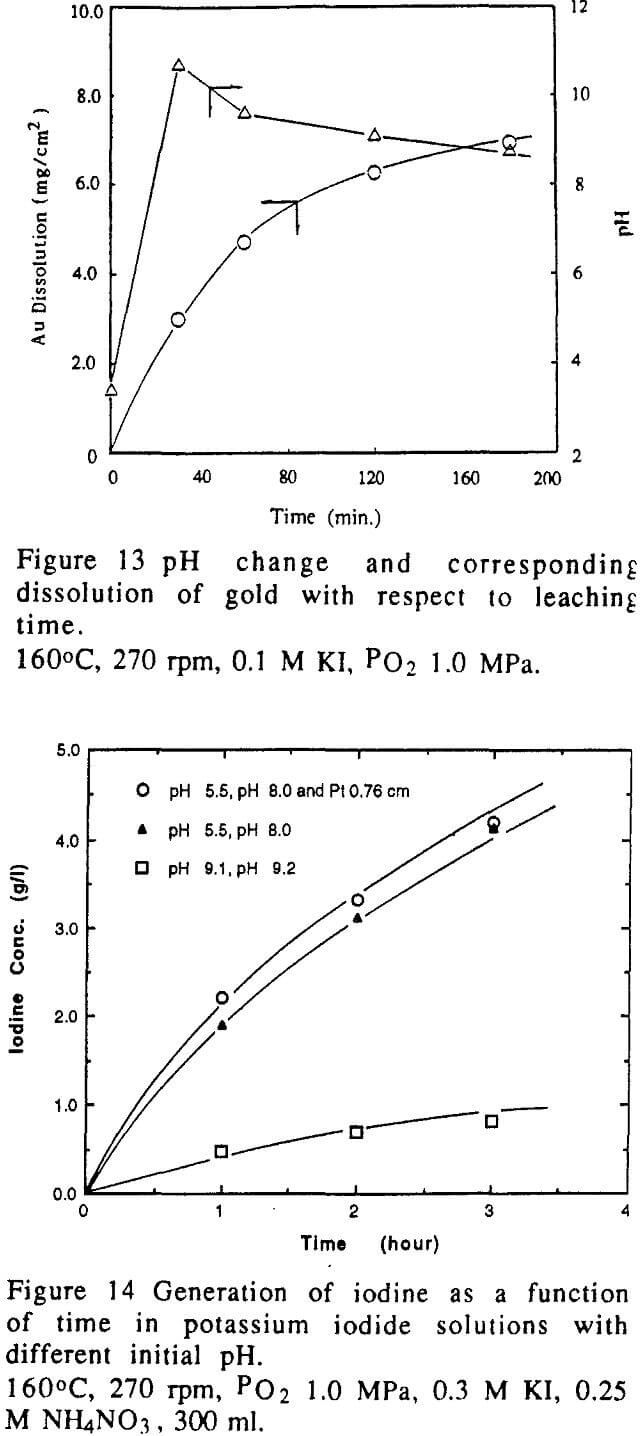
It is well known that the cyanide solution is very toxic. The environmental concerns resulting from the use of cyanide have been an industrial problem. Therefore, intense efforts have been made over the last decade to find alternative lixiviants for gold leaching. Thiourea, thiosulfate, halides and ammonia have been investigated and evaluated as gold lixiviants. The authors of this study have recently applied pressure leaching with iodide to refractory gold ores, a technique developed as a noncyanide process for refractory gold ores. This new process is a one stage operation, where the oxidation of sulfide and the dissolution of gold are completed in an autoclave within 2-4 hours. Because of this advantage, the process is believed to be technologically and economically viable for refractory ores.
The use of iodide to recover gold from gold bearing materials has been reported in literature. In these previous studies, elemental iodine was essential not only as an oxidant but also as a lixiviant. The fundamentals of such an iodine leaching at ambient temperature was studied. However, the investigation on the dissolution behavior of gold in iodide solutions at elevated temperatures, such as the case in the system considered here has not yet been reported.
Experimental
Batch leaching experiments were carried out in a 1000 ml autoclave made by Parr Instrument Co. The temperature was controlled within ±5°C and the stirring speed was controlled. One piece of high-purity gold square plate with total surface area of 2.95 cm² was used in each leaching experiment. The gold plate was suspended in the leach liquor when the experiment was carried out.
Leaching solution of 300 ml was used in each experiment and was prepared by dissolving known amounts of reagent grade chemicals in distilled water. Before leaching experiment was conducted, gold plate and leaching solution were placed in the autoclave. The autoclave was sealed and heated to a temperature about 30°C below the desired temperature. Then pure oxygen was injected into the autoclave until the desired pressure was achieved. The desired temperature was rapidly reached, due to the exothermic reactions in the system. Usually, this course takes 3-5 minutes. The leaching time was taken into account when oxygen was inserted. About 20 ml liquid samples were withdrawn at various time intervals. The gold concentration of the solution sample was analyzed by a Perkin-Elmen atomic absorption spectrophotometry. The volume change caused by sampling and Au in the sample solutions were taken into account, when the calculations of the Au dissolution were made. The concentration of iodine was analyzed by titration with sodium thiosulfate solution.
Leaching experiments were carried out at 160°C, with 350 rpm of stirring speed. The standard error of leaching experiments was less than 4%.
Results
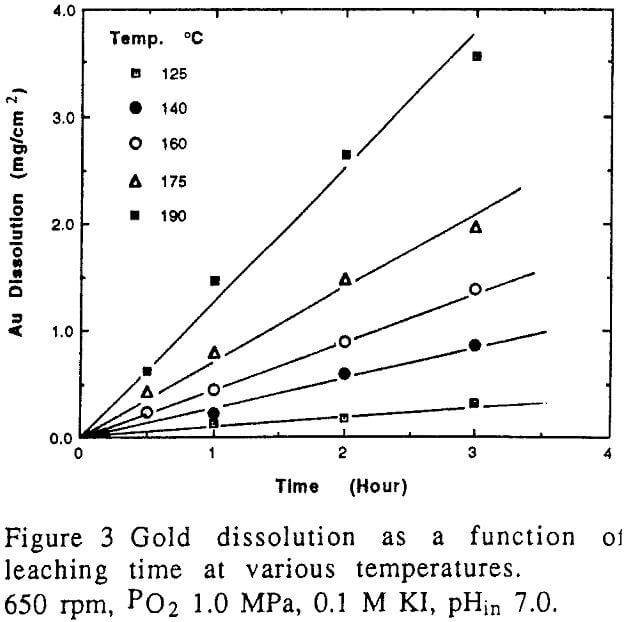
Gold can dissolve in iodide solutions in the presence of an oxidant even at room temperature. However, experimental results conducted in this study and reported in the literature indicated that dissolution of gold in iodide solution with oxygen at ambient temperature was so insignificant that the dissolution is impractical. Based on the kinetic principles, the dissolution of gold will be remarkable at elevated temperatures. Figure 3 shows the typical results of experiments conducted at high temperature in an autoclave. It is obvious that an increase in the leaching temperature results in the increase of dissolution rate of gold. This dissolution exhibited a linear relation with the leaching time within 3 hours. This behaviour can be described by the kinetic mode described earlier. Therefore, the dissolution rate of gold was obtained from the slope of the straight line given in Figure 3. In the light of Equation 8, the factors such as hydrodynamics, temperature and concentrations of reactants will have influence on the dissolution rate of gold.
Effect of stirring speed
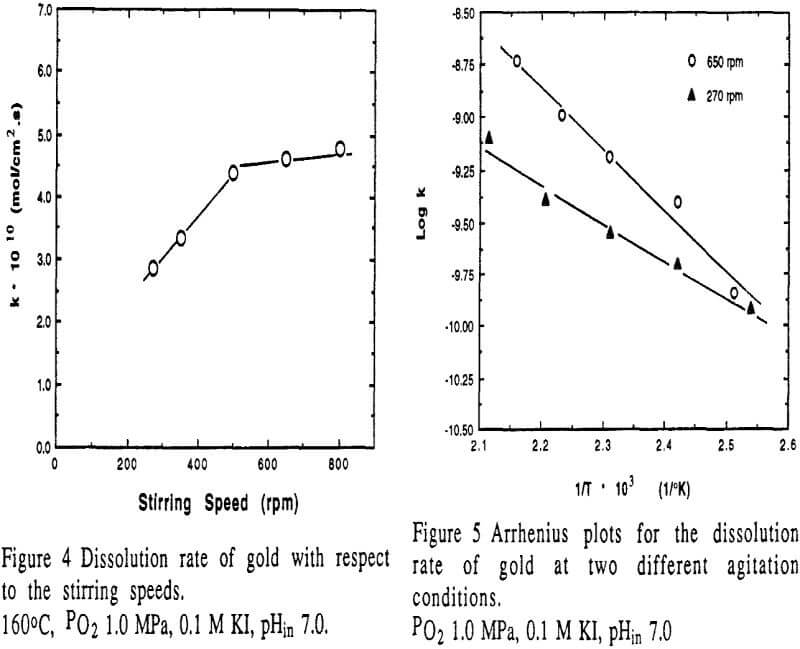
The dissolution rate of gold with respect to the stirring speed under the specified conditions. It can be seen that low stirring strength under 500 rpm has an effect on the rate. However, the effect of stirring speed above 500 rpm is insignificant.
Effect of temperature
The effect of temperature on the dissolution of gold is significant.
Effect of partial pressure of oxygen
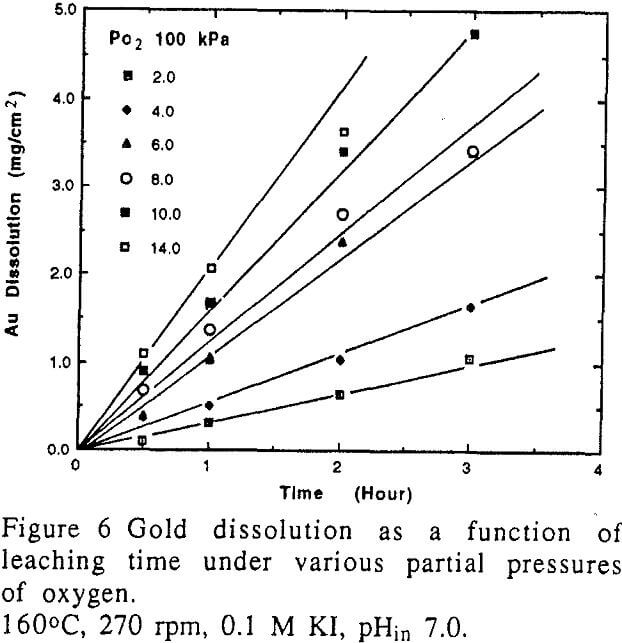
Theoretically as well as practically, gold can not dissolve in iodide solution in the absence of an oxidant. Oxygen is an inexpensive and effective oxidant. The rate of gold dissolution is proportional to the partial pressure of oxygen in the range used in the study.
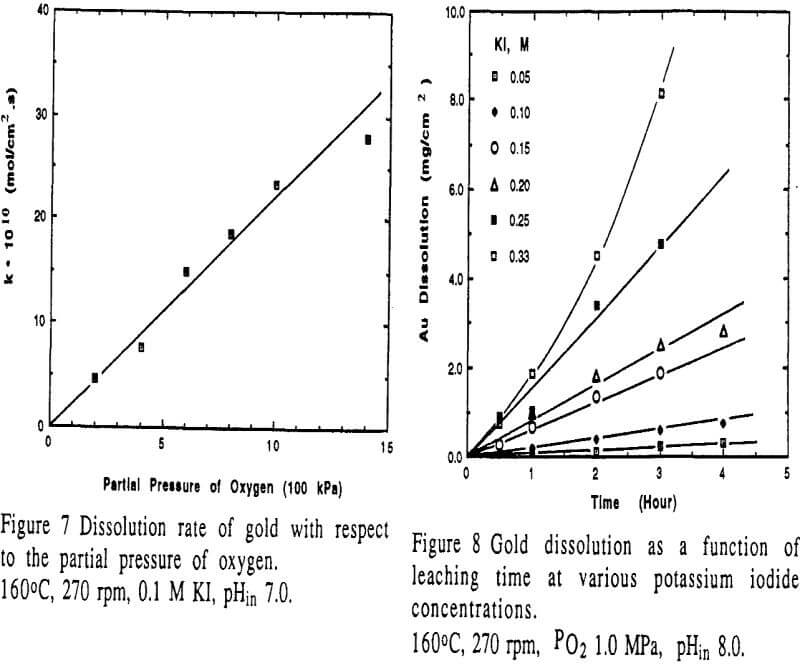
Effect of potassium iodide concentration
Potassium iodide was used as a lixiviant in this study. Chemically, iodide ion acts as a reactant for the dissolution of gold. Iodide concentration has a remarkable effect on the dissolution of gold. The rate is proportional to the square of potassium iodide concentration.
Effect of pH of iodide solution
The effect of pH on the dissolution rate was examined through experiments conducted in various potassium iodide solutions containing different electrolytes. The pH of the iodide solution was adjusted with 0.1 M H2SO4, NaOH or NH4OH, before a leaching solution was put into the autoclave. It was noted that the pH of the leaching solution changed during the course of dissolution.
The dissolution rate of gold was a function of the initial pH of the leaching solution. At the low range of pH, the rate increased very sharply as the pH of the solution decreased. 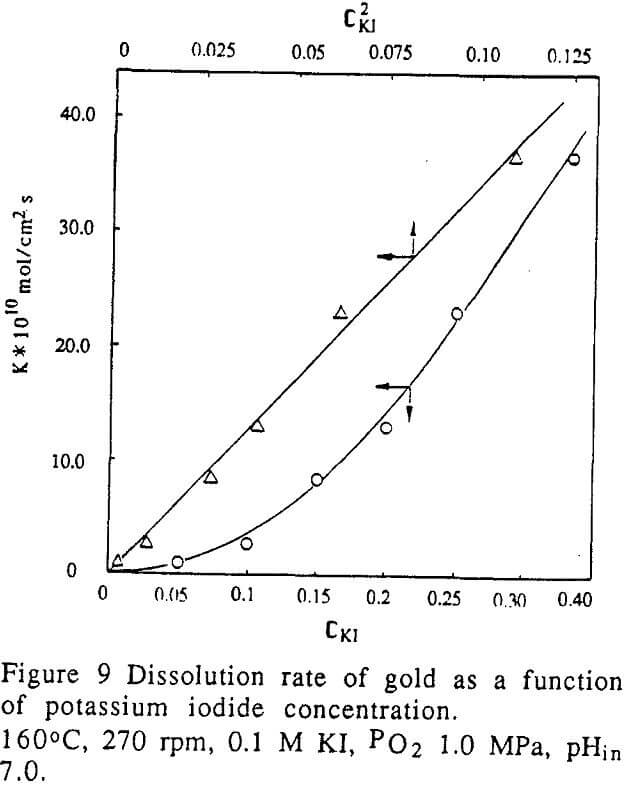
In the range of pH 6-9, the rate was zero order against pH.
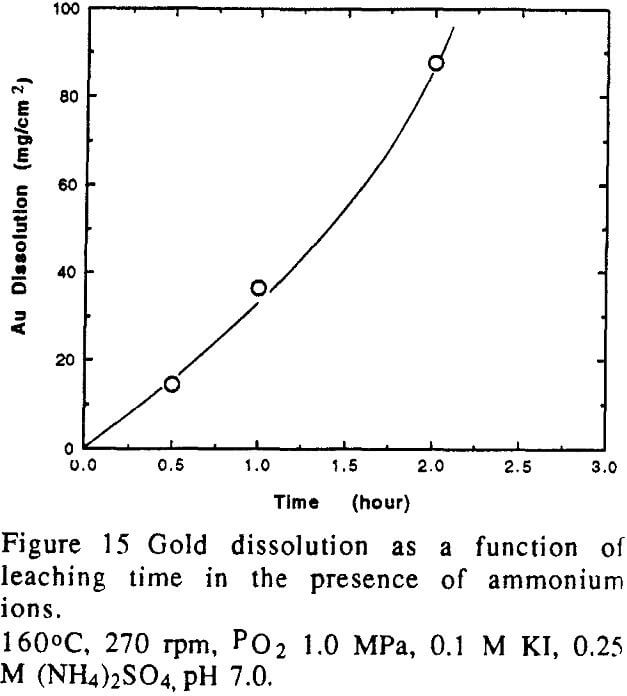
It was interesting to observe that when the dissolution of gold was carried out in iodide solutions containing ammonium ions, the dissolution rate increased dramatically. The rate improved by about 100 times in the presence of ammonium ion at the optimum pH. This suggests that the ammonium ion had a catalytic effect on the dissolution of gold in iodide solutions.
Conclusion
- The dissolution of gold in iodide solutions with oxygen is described by means of a kinetic model, which includes the contribution of heterogeneous reaction and mass transfer of reactant and product. The mass transfer of reactant and product has little contribution to the dissolution rate of gold.
- The dissolution mechanism has much to do with the oxidation of iodide to iodine, because iodine was found during the reaction process and the dissolution rate increased substantially when iodine presented in the iodide solution.
- Under experimental conditions, the heterogeneous reaction is the dominant limiting step. The dissolution rate of gold is second order with respect to iodide concentration, and first order to the partial pressure of oxygen. The apparent activation energy is found to be 56.3 kJ/mol at higher stirring conditions and 34.7 kJ/mol at lower stirring.
- A proton-providing ion such as hydrogen ion and ammonium ion can greatly accelerate the oxidation of iodide to iodine, resulting in a great deal of dissolution of gold. A oxygen-transferor ion such as Cu²+ and Fe³+ can also accelerate the oxidation of iodide and consequently increase the dissolution of gold.

