Table of Contents
Germanium is an important strategic metal used in fiber optics, infrared detectors, and semiconductor devices including transistors, diodes, and rectifiers. Substitution for germanium cannot be made without substantial loss in performance in most cases.
Germanium is generally not concentrated naturally, although trace amounts are found in many minerals. Germanium is produced almost exclusively from waste residue of hydrometallurgical zinc processing operations (Llewellyn, 1989). Currently, high-grade domestic zinc residues containing enough germanium to supply the annual needs of the U.S. are processed in foreign countries by proprietary techniques to recover the germanium (Plunkert, 1985). The Bureau of Mines, in an effort to enhance domestic processing capabilities, has developed methods of recovering germanium from these residues.
Previous work (Harbuck, 1988-89) showed that even under optimum leaching conditions (1000 kg/mt H2SO4, 5% solids, and 80°C), only 70 to 80% of the germanium was extracted from these zinc residues. To increase germanium extraction, additional research identifying the mineralization of this “insoluble” germanium has been conducted. Previous data (Harbuck, 1988-89) indicated that the “insoluble” germanium was present in solid solution with silica. It was theorized that this solid solution formed in the roasting stage of zinc processing. In this paper, a hypothesis is presented explaining how germanium becomes tied up with silica during the leaching stages of zinc processing.
Material and Equipment
A domestic zinc company supplied the zinc calcine (ZnO, formed at approximately 1000 to 1100°C) and process residue used in this research. Elemental analyses of these materials, as determined by inductively coupled plasma spectrophotometry (ICP), neutron activation analysis (NAA), and wet chemical methods, are given in Table 1. X-ray diffraction (XRD) and scanning electron microscopy (SEH) revealed that the zinc calcine was primarily ZnO, while the process residue contained ZnS, CaSO4, PbSO4, SiO2 , FeS, ZnFe2O4, and free sulfur. The concentration of germanium was so low and the particle size so fine, that SEM could not detect discrete germanium-bearing minerals in either sample.
Agitation leach tests were performed at various experimental conditions using standard laboratory hotplates, thermocouples, stirring devices, and glassware. Roasting tests were performed in a vertical tube furnace arranged for continuous operation (unless otherwise noted).
Understanding Germanium Insolubility
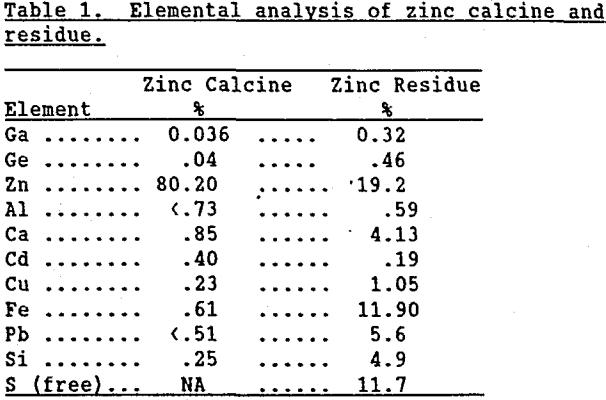
To better understand how germanium becomes insoluble in zinc residue it is important to review the stages of zinc processing. A process flowsheet shown in Figure 1 depicts the major operations:
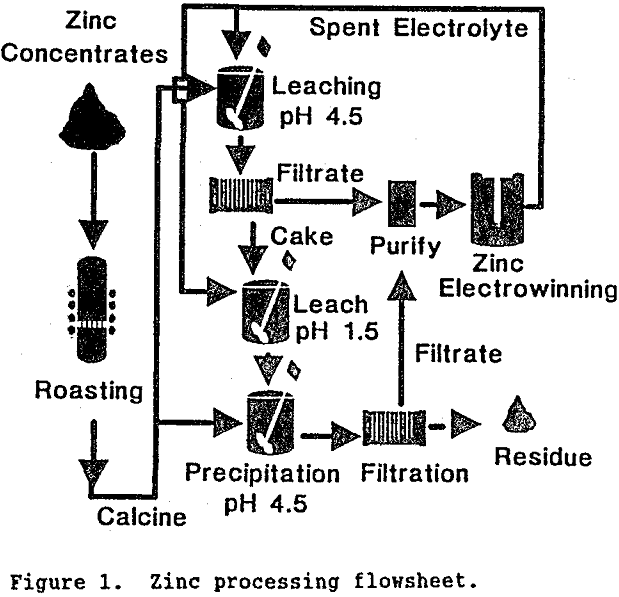
1) Roasting of sphalerite (ZnS) to calcine (ZnO); 2) “Neutral” leaching of approximately 70% of the calcine at pH 4.5 to form a ZnSO4 solution which is further purified and sent to zinc electrowinning; 3) “Weak acid” leaching of the “neutral” leach residue to dissolve remaining zinc; and 4) Neutralization to pH 4.5 of “weak acid” leach solution to precipitate impurities from the ZnSO4 solution before it proceeds to further purification and zinc electrowinning. The hypothesis and experimental verification of changes germanium undergoes in each of these steps is described in the following text.
Zinc Processing Steps
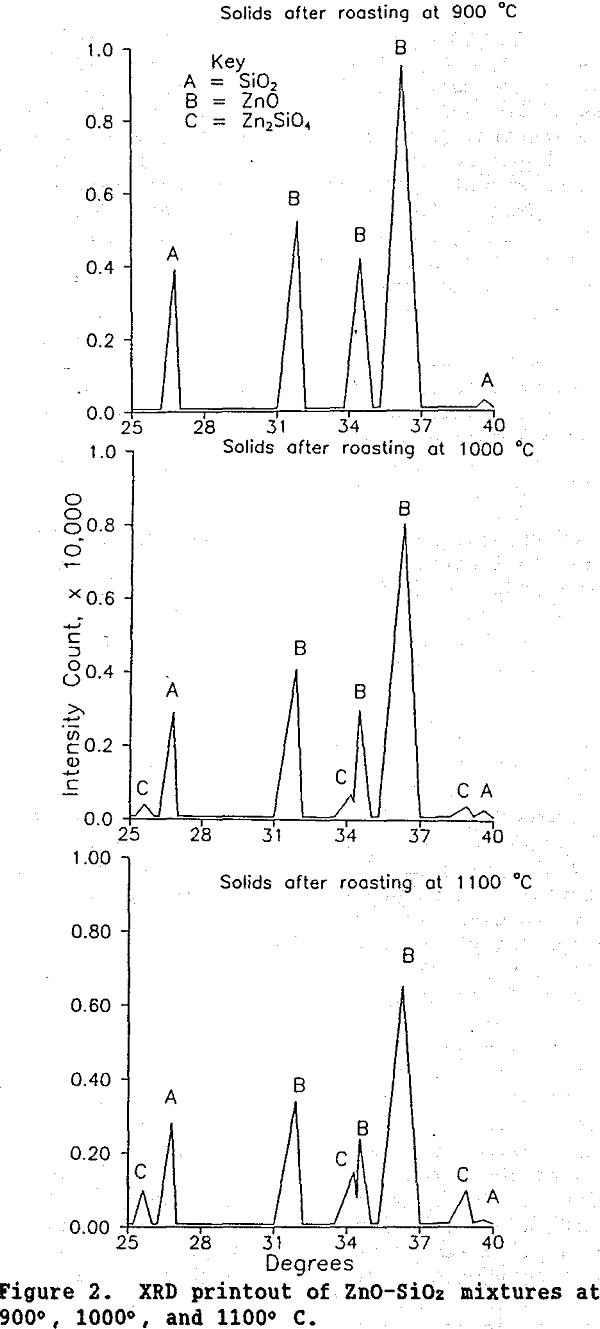
Roasting: Zinc concentrates containing minor amounts of Fe, Cu, Pb, SiO2, Ga, and Ge are roasted at 900° to 1,100°C to convert ZnS to ZnO. It is generally believed that the germanium associated with sphalerite is primarily in a sulfide form (Brewer, 1955; Shcherbina, 1987). During roasting, germanium sulfide is converted to GeO2. Previous work at the Bureau of Mines (Harbuck, 1988-89), along with research by Umetse and Tozawa (1987), showed that at a roast temperature of 1,000°, greater than 90% of the GeO2 present reacts with ZnO according to reaction A.
GeO2 + 2 ZnO = Zn2GeO4………………………………………………..(A)
Another important reaction occurring during roasting is the combining of ZnO with SiO2 to form Zn2SiO4, reaction B.
2 ZnO + SiO2 = Zn2SiO4………………………………………………(B)
Reaction B was verified by uniformly mixing and then roasting stoichiometric amounts of SiO2 and ZnO at 900°C for 10 h in a horizontal tube furnace. This procedure was repeated for roasts at 1,000° and 1,100°C. An examination of the reaction products using XRD revealed that Zn2SiO4 began to form at 1,000°C (Figure 2). After roasting at 1,100°C, a substantial amount of this compound was present.
Leaching: In the two-stage leaching process employed by the zinc industry (Figure 1), approximately 70% of the zinc calcine is dissolved after 5 to 6 h in a pH 4 to 5, H2SO4 solution (“neutral” leach). When the slurry from this leach is filtered, the solution is further purified before proceeding to zinc electrowinning. The solids are re-leached in a stronger H2SO4 solution (pH 1.5 “weak acid” leach) at 60°C for 2 to 3 h to dissolve the remaining zinc. Typical analyses for both of these leach solutions are given in table 2.
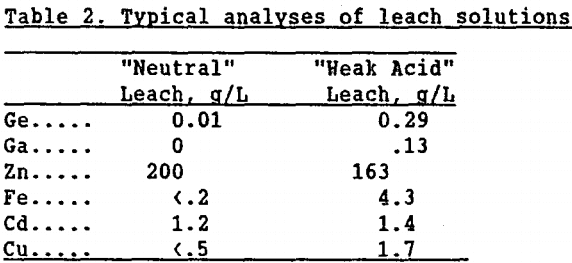
As seen, most impurities, including germanium, are solubilized in the “weak acid” leach, indicating the dissolution of Zn2GeO4 at these conditions. Zangieve, et al. (1983), verified the dissolution of Zn2GeO4 in similar pH ranges. The dissolution of Zn2SiO4 was tested using the two-step leach process on commercially-prepared material. In the “neutral” leach, approximately 20% of the Zn2SiO4 dissolved, while 97% of the remaining Zn2SiO4 dissolved in the pH 1.5, “weak acid”
leach. Matthew and Elsner extensively studied the leaching of zinc silicate ores with H2SO4 (Matthew, 1977a; Matthew, 1977b). They discovered that Zn2SiO4 dissolves to form Si(OH)4 and zinc sulfate according to reaction C at a pH of approximately 1.5 to 2.
Zn2SiO4 + 2 H2SO4 = 2 ZnSO4 + Si (OH)4………………………………..(C)
The Si(OH)4 polymerizes to produce polysilicic acid. With continued polymerization, the polymers attain colloidal dimensions. The stability of colloidal silica particles is dependent on such factors as pH, temperature, concentration, and ionic strength: Unstable colloidal particles aggregate to form silica gel.
It is postulated that in the “weak acid” leach, where both solubilized germanium and silica gel are present, a portion of the germanium becomes tied up with silica gel.
Gruzdev and Vydrin (1967) documented the affinity germanium has for silica. Miller, et al. (1963) demonstrated how easily GeO2 can substitute into the four-fold coordinated structures of silica. Caletka and Kotas (1974) observed that silica gel selectively adsorbed germanium from acidic solutions. In the present investigation, commercially prepared -3mm silica gel was contacted with a pH 1.5 H2SO4 leach solution containing 0.44 g/L Ge as well as Ca, Cd, Cu, Fe, Zn, and Ga. As seen in Table 3, the silica gel selectively removed germanium from solution.
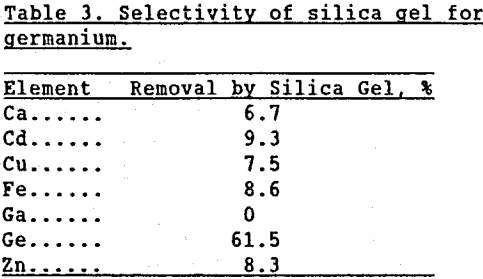
The hypothesis that germanium becomes tied up with silica in the “weak acid” leach was further verified by adding varying amounts of Zn2SiO4 to zinc calcine and leaching the mixture in the two-stage zinc leaching process. Less than 2% of the germanium was solubilized in the “neutral” leach. The slurry from the “weak acid” leach was filtered and the solids were then leached five separate times in 20% H2SO4 solutions (this procedure will further be referred to as “intensive” leach) to ensure that all the soluble germanium was removed. Table 4 shows that as the amount of added Zn2SiO4 increased, the amount of soluble germanium in the “weak acid” leach residue decreased. When enough Zn2SiO4 was added to correspond to 10 wt % SiO2 in the calcine, germanium extraction from the “weak acid” leach solids decreased to below 38%. Gallium extractions are included to emphasize that only the germanium was insolubilized.
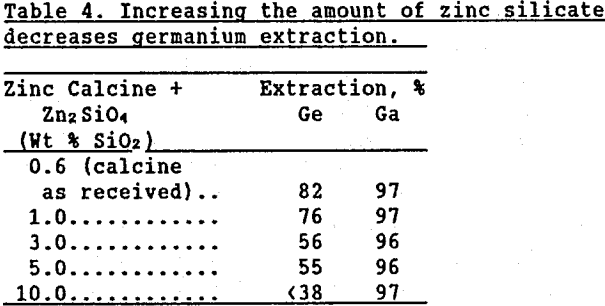
These results suggest that Zn2SiO4 dissolved to form silica gel which removed germanium from solution. As additional evidence, the 10 wt % SiO2 residue (remaining after the “intensive” leach was completed), was examined with both a standard laboratory microscope and a cross polarized light microscope. Because silica gel is isotropic, it is not visible in cross polarized light. The residue could easily be seen using the standard microscope. However, under cross polarized light, most of the residue was not visible, indicating the presence of silica gel.
This same residue was also examined using XRD. Because XRD is a technique used to identify crystalline solids, silica gel, being amorphous, should produce no XRD pattern. The XRD background pattern of the residue from the zinc calcine-Zn2SiO4 mixture drifted upward and the compound peak heights were substantially diminished compared with the XRD pattern of the residue produced without the addition of Zn2SiO4. This phenomenon indicates that the residue produced with Zn2SiO4 addition was less crystalline than the residue produced without Zn2SiO4 addition. This further supports the hypothesis that silica gel was formed.
Neutralization: Following the “weak acid” leach, calcine is added to the slurry to precipitate impurities and raise the pH to approximately 5. The germanium in solution which is not bound to silica is precipitated with the other impurities. Testing showed that this precipitated germanium is readily soluble in H2SO4 solutions. The silica gel with adsorbed germanium proceeds through the purification step unaffected and is carried along in the precipitate-gangue mixture.
In summary, by using current leach practice, approximately 20 to 30% of the germanium reporting to the zinc residue is tied up with silica gel. Even under “intensive” H2SO4 leaching conditions, this germanium will not be solubilized.
Possible Solutions to the Problem
Potential alternatives for increasing germanium extraction were investigated. Methods tested, together with research results include the following:
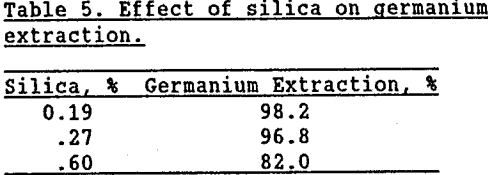
Lower the silica content of zinc concentrates before they enter the roaster. If less silica is present, then less Zn2SiO4 will form and more of the germanium will be recoverable from subsequent process residues. Silica removal was explored using a Bureau of Mines column flotation system (Brewer, 1955). Zinc sulfide concentrates containing 0.6% silica (obtained using traditional flotation cells) were reprocessed using column flotation to obtain concentrates containing 0.19 and 0.27% silica. (Greater than 96% of the zinc was recovered and greater than 70% of the calcium and magnesium were eliminated.) The “clean” concentrates were then roasted at 1,000°C. The calcines produced were leached by two-stage zinc process leaching methods. The residues from these leaches were then “intensively” leached to remove all soluble germanium. As seen in Table 5, decreasing the silica content in the concentrates prior to roasting increased the amount of germanium which could be extracted from the subsequent zinc residues.
Lower the temperature of the roast. As shown in Figure 2, at lower roast temperatures, less Zn2SiO4 is formed. If less ZnzSiO4 is present, less silica gel will form to insolubilize germanium.
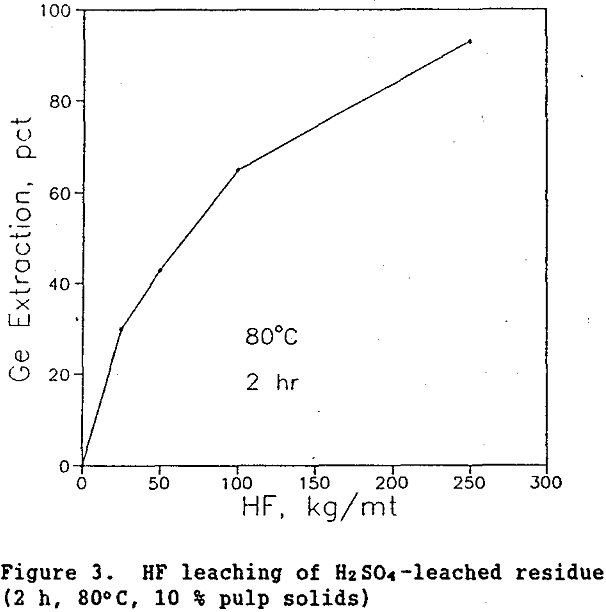
Leach the insoluble germanium in the residue with HF acid. It is well known that HF dissolves silica. Since the germanium is tied up with silica, leaching with HF will free the germanium. Figure 3 shows germanium extraction versus HF addition for zinc residue which had previously been leached with H2SO4. As seen, leaching the H2SO4-leached residue with 250 kg/mt HF for 2 h at 80°C and 10% pulp solids extracted 93 % of the remaining germanium, bringing total germanium extraction from approximately 70 to 98%. (The high cost of HF could make this alternative less viable.)
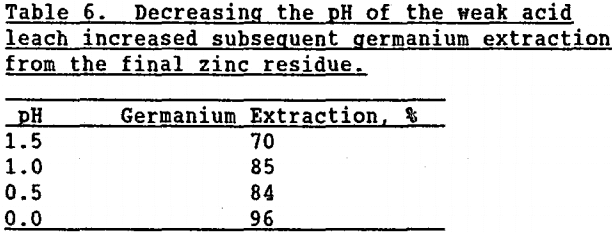
Modify the two-stage zinc leaching procedure. Testing showed that silica gel formation in the “weak acid” leach was affected by solution pH. Table 6 demonstrates that as the pH of the “weak acid” leach decreased from 1.5 to 0, germanium extraction during subsequent leaching of the final zinc residue increased from 70 to 96%.
Summary
Some hydrometallurgical zinc residues contain a significant amount of germanium; however, only 70 to 80% of the germanium can be recovered by H2SO4 leaching. Bureau of Mines test work indicated that with current practice, 20 to 30% of the germanium becomes insoluble during the leaching stages of zinc processing. Minor amounts of zinc silicate present in zinc calcine dissolve during leaching to form silica gel and zinc sulfate. Germanium is selectively removed by this gel. Several potential alternatives exist to avoid this problem:
- Remove silica from the concentrates by column flotation.
- Perform zinc sulfide roasting at a lower temperature to avoid formation of zinc silicate.
- Leach the insoluble germanium with HF acid.
- Decrease the pH of the “weak acid” leach to avoid losing germanium in silica gel formation.
