Table of Contents
- Description of Process
- Byproduct Removal from Crude Titanium Chips
- Summary of Operation
- Sources and Effects of Impurities
- Removal of Atmospheric Contaminants from TiCl4
- Prevention of Atmospheric Contaminants in Reduction Operation
- Removal of Atmospheric Contaminants in Vacuum Distillation Operation
- Minimizing Moisture Contamination in Process
- Moisture Contamination in TiCl4 Purification System
- Dehydration of Air for Pumping Crude HCl4
- Effect of Moisture on B.h.n. of Titanium Sponge
- Safety Hazards
- Determination of Moisture Contamination in TiCl4
- Moisture Contamination in Dryroom
- Theoretical Aspects in Dehydration of Magnesium Chloride
- Oxygen Contribution of Magnesium Oxide
- Dehydration Techniques in Reduction Operation
- Dehydration Techniques in Vacuum Distillation Operation
- Results of Dehydration Tests
- Correlation Studies on Moisture and B.h.n.
- Effect of Lower Chlorides on B.h.n.
- Control of Other Impurities in Titanium Sponge
Research revealed that by systematic elimination of detrimental impurities introduced with the raw materials and by improved handling and processing techniques, high-quality ductile titanium with a hardness of less than 110 B.h.n. (Brinell hardness number) can be produced consistently. This improvement was accomplished by helium (or argon) stripping of residual atmospheric and other dissolved gases from the titanic chloride, preliminary dehydration of crude titanium sponge, and careful regulation of the titanium tetrachloride (TiCl4) feed rate to the reactors.
Results were achieved by adapting existing equipment in the titanium pilot plant. Tests indicated that even softer sponge could be produced by redesigning the plant-processing equipment, particularly to accommodate larger batches in each operation.
In the hydrogen sulfide (H2S) treatment of TiCl4, the sulfiding temperature was increased from 90° to 115° C. When the addition of hydrogen sulfide was completed, the 115° C. temperature was maintained while a volume of helium (or argon) equivalent to that of the sulfiding vessel was bubbled through the liquid. The dissolved gases, unreacted hydrogen sulfide gas, and low-boiling impurities were removed from the TiCl4. Titanium sponge made from the purged TiCl4 had a hardness of 112 Brlnell, compared with 134 Brinell for metal made from the unpurged TlCl4.
Moisture contamination was effectively minimized by preheating the reduction vessel under a vacuum of 27 to 28 inches mercury at 300° C. for 1 hour. Likewise, moisture contamination in the distillation retort was reduced by preheating the charge of crude titanium chips at 205° C. for 2 hours. The retort waa backfilled with helium (or argon) before starting the vacuum distillation cycle. This preheat and inert gas-purge treatment in both the reduction and distillation vessels removed some of the water picked up by the magnesium chloride (MgCl2). By the preheating treatment the average B.h.n. of the resultant sponge metal was reduced from 112 to 100.
Other steps that helped to improve the titanium-sponge product are summarized as follows:
- An inert gas blanket was maintained at all times to protect the TiCl4 purification system from exposure to air.
- A glass-wool filter installed in the purified TiCl4 feed lines removed solid entrained particles such as iron, vanadium, and aluminum compounds.
- The absence of lower chlorides of titanium was usually noted in the best grade of sponge titanium. Careful regulation of the feed rate of HCl4 to prevent “force feeding”, particularly at the end of the reduction reaction, minimized formation of the lower chlorides of titanium. These compounds, if formed, are detrimental to titanium sponge in that they are very hygroscopic and pyrophoric.
- During the vacuum distillation cycle, a point is reached when the vacuum developed in the retort exceeds the vacuum created by the mechanical pump. Closing the valve between the retort and the pump at the proper time prevented back-diffusion of oil vapors, which would contaminate the titanium sponge in the retort. A diffusion pump of sufficient pumping capacity in the vacuum system would eliminate the need for the foregoing step and improve the removal of magnesium and magnesium chloride entrapped in the sponge metal.
Based on observations made during this investigation, here are two additional steps that can be taken to effect further reductions in the impurities in titanium sponge:
- Although there was no significant difference in the hardness of titanium sponge made with various grades of magnesium, it was apparent that certain impurities in the sponge might be reduced by using high-purity magnesium.
- In the dryroom, where the raw titanium sponge is bored out of the reduction vessel, air-drying equipment should have sufficient capacity to maintain the dewpoint at a minimum of -40° F. The best quality sponge was produced under that condition. However, need for a dryroom can be eliminated by designing equipment that combines reduction and distillation operations in the same vessel.
Since W. J. Kroll first devised a method for producing ductile titanium, the Bureau of Mines has developed the process from a laboratory scale of several grams to a pilot-plant scale of 200 pounds. Successful development of the Kroll magnesium-reduction process by the Bureau led to establishment of a domestic titanium industry.
In 1952 the Army Ordnance Corps provided funds for erecting a pilot plant at Boulder City, Nev., with a production capacity of 1,500 pounds of titanium sponge per day. The larger scale plant was to be used to effect process improvements and to develop a continuous process. In 1953 research showed that the Kroll process was difficult, if not impossible, to adapt to continuous operation, and the plant was used for a limited-production research program under contract with the General Services Administration (GSA).
Design and operational details of this pilot plant are described in two Bureau publications. Production and cost figures for the operation are published in another report.
Besides being used for proving process developments that required a continuously operating plant for proper evaluation, the pilot plant served as a demonstrator for many industrial representatives who had started production of titanium, or who were evaluating the possibilities for such production.
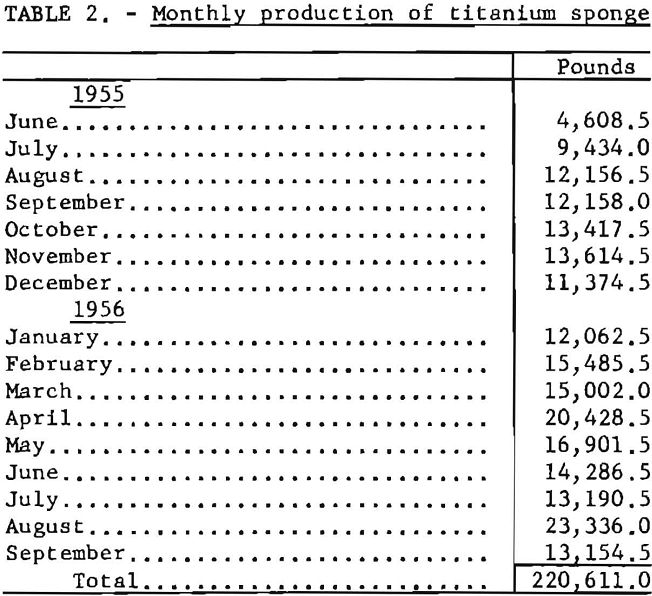
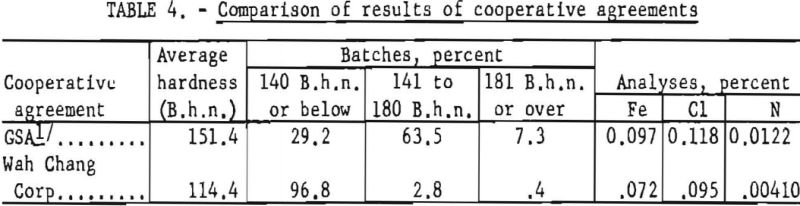
In May 1955 the Wah Chang Corp., of New York, and the Bureau of Mines entered into a cooperative agreement to conduct research and development on the production of improved titanium sponge metal at the Boulder City plant. Bench-scale research results obtained in the Wah Chang’s laboratories at Glen Cove, N. Y., had indicated the desirability of further development work on a pilot-plant scale. From June 1955 through September 1956, a total of 220,611 pounds of titanium sponge was produced. Average hardness of the metal was 114 B.h.n. Under optimum conditions, batches were produced with a hardness below 110 B.h.n. and as low as 90 B.h.n.
Description of Process
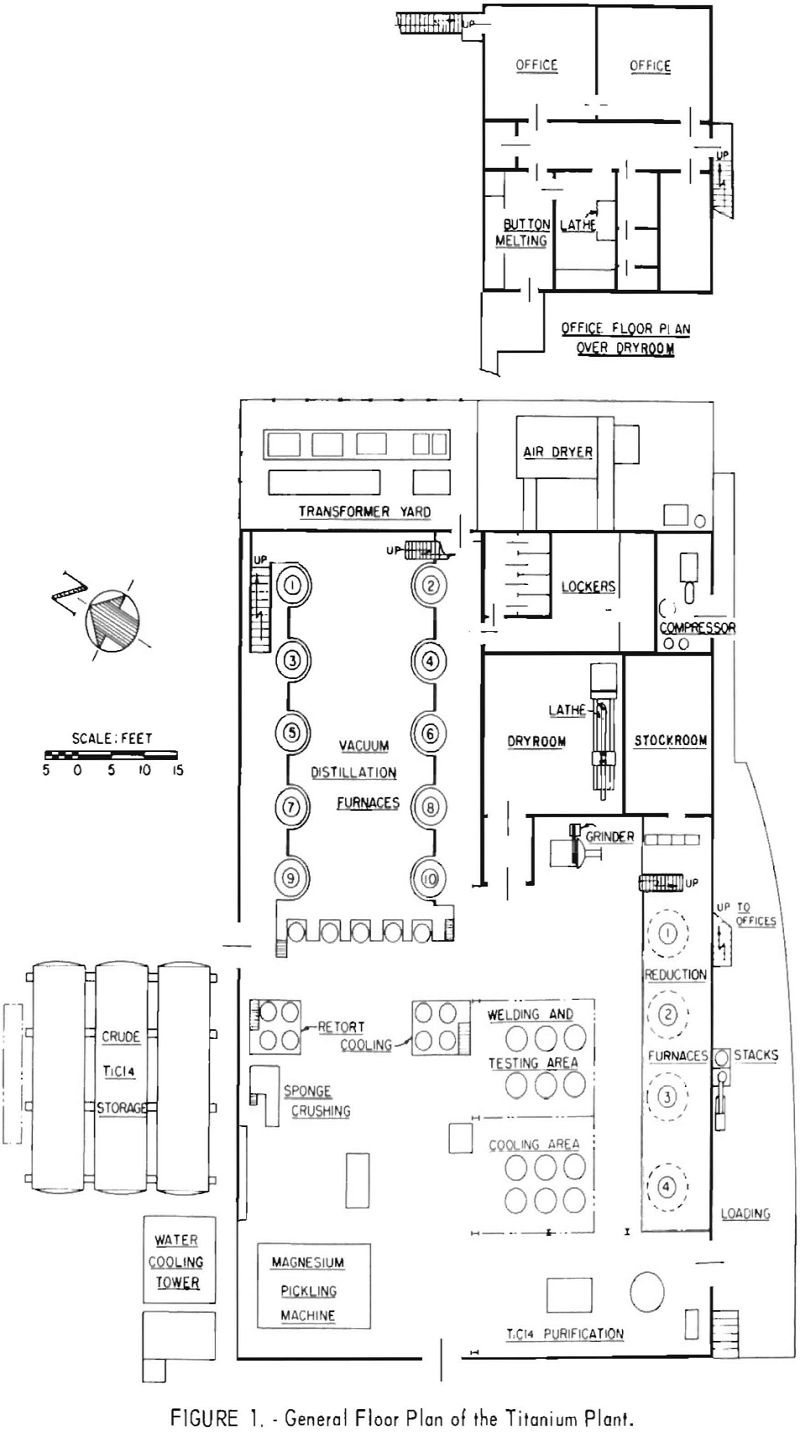
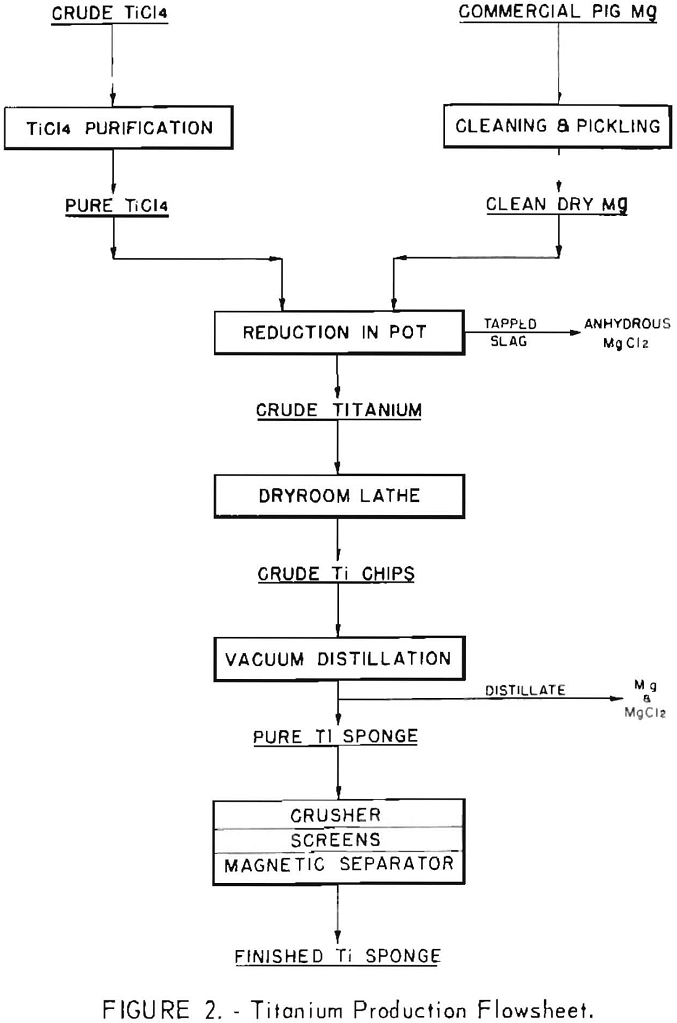
The general layout of the plant, and a detailed description of the processing equipment and operating procedures have been given in a previous Bureau of Mines report. The general layout of the pilot plant and a simplified flowsheet are shown in figures 1 and 2, respectively.
The starting materials were crude TiCl4 and Mg, both purchased from commercial producers. Although the titanium sponge produced was analyzed chemically, the measure of quality of the metal was based primarily on hardness, expressed as B.h.n. The technique and equipment used for determining tita¬nium sponge hardness are described in a Bureau report.
Purification of TICl4
Some of the common impurities found as dissolved compounds in crude TiCl4 are oxgyen, nitrogen, carbon dioxide, chlorine, hydrochloric acid, silicon tetrachloride, silicon oxychloride, carbon tetrachloride, chloroacetyl chlorides, carbonly chloride or phosgene, sulfur chlorides, vanadium oxychloride, aluminum chloride, and ferric chloride. Solid particles, usually finely divided or colloidal, may also be present. These particles may include hydrolysis products of titanium and silicon, carbon, ferric chloride, and various metallic oxides in trace quantities.
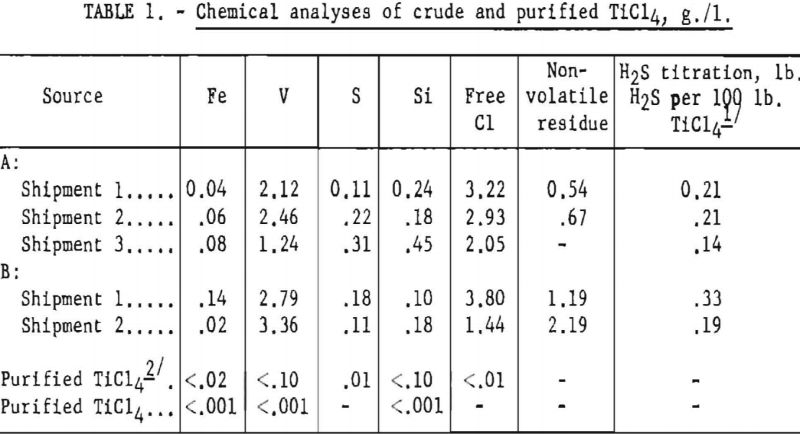
Sample analyses of several tank carloads of the crude TiCl4 used in the operation are listed in table 1. The table also shows typical analyses of the purified product.
Purification of the crude TiCl4 was accomplished in five steps: (1) Treatment with hydrogen sulfide to precipitate metallic compounds, particularly those of vanadium; (2) helium (or argon) stripping to remove dissolved gases; (3) fractional distillation to remove low-boiling impurities; (4) distillation of TiCl4 from precipitated residues; and (5) filtration of the purified TiCl4.
Crude TiCl4 was heated to 115° C., and a total of about 7.5 pounds of hydrogen sulfide (H2S) was added at the rate of 1-½ pounds per hour for each batch of 1,350 to 1,400 pounds of TiCl4. The amount used was that required to give a colorless liquid.
Vanadium compounds could be removed by a number of substances, including sodium amalgam, various fatty acids and soaps, finely divided copper powder, and hydrogen sulfide. Hydrogen sulfide is preferred because the products can be removed readily from the sulfiding vessel.
The sulfiding vessel was a closed mild-steel vessel, 30 inches across and 40 inches deep. A high-speed stirrer was inserted through a 5-inch flange fitting on top of the vessel. The hydrogen sulfide inlet was just above the impeller, which revolved inside a short length of pipe (open at both ends) and discharged downward, breaking the gas into small bubbles and mixing them with the liquid. Three electrical Immersion heaters mounted in vertical convection tubes at the side of the tank were used to heat the contents. Each heater had a capacity of 2,000 watts, and all were protected, when not completely immersed, by a low-level, cutoff float switch. A thermostat held the temperature to any desired value below the boiling point of TiCl4.
After the required amount of H2S had been added, helium (or argon) was introduced for 1 hour at the rate of 50 c.f.h. (cubic feet per hour) while the temperature was maintained at 115° C. After this stripping operation, about 99 percent of the dissolved gases had been removed.
The charge was transferred next to the fractionating still, where the TiCl4 was brought to boil. The vapors were refluxed in a column packed with Raschig rings, and noncondensable fractions were vented from the system. The fractionating cycle was complete when approximately 3 percent of the charge of TiCl4, termed “lights”, was condensed and collected in a lights storage tank. When a full tank had been collected, the lights fraction was reprocessed. There was no significant difference in the harndess of titanium sponge made from purified commercial TiCl4 and that made from reprocessed lights.
The TiCl4 slurry and sulfide-precipitated solids were then transferred to a still, where TiCl4 was distilled away from the solid impurities at a maximum rate of 350 pounds per hour. The distilled TiCl4 was collected in storage tanks kept under slight helium pressure.
Finally the TiCl4 was passed through a filter packed with glass wool and fed to a reduction pot. All filtrable impurities, primarily of vanadium and iron, were removed by the glass-wool packing.
Reduction of Purified TiCl4
The purified TiCl4 was reduced by magnesium in a pot-type reactor (reduction pot) to form spongy titanium and a magnesium chloride byproduct.
Magnesium in the form of notched ingots was pickled in dilute muriatic acid to remove surface oxide film and flux inclusions. The pickled magnesium was rinsed thoroughly, dried in an oven, and moved to the dryroom for storage until ready for use.
The dryroom is the starting and the end point In the reduction cycle. Here the reactor pots are filled with magnesium ingots, the lids of the pots are put on, and the joints between the lids and the pots are clamped tightly and taped before going to the welder. Later the reaction-mass contents of the pots are turned out on a lathe into chip baskets.
Since the B.h.n. of titanium metal is affected largely by the oxygen content of the metal, and since moisture contamination contributes oxygen, it is extremely important that the crude titanium mass be protected from accumulating water of hydration. Thus the reactor pot is opened, charged, and discharged only in a room containing air of very low humidity the dryroom. The design and operation of the dryroom are described in another Report of Investigations.
After the reduction pot had been charged in the dryroom with a predetermined weight of magnesium (generally about 330 pounds per pot), the pot lid was welded in place. When all leaks had been sealed, the pot was placed in the reduction furnace set at 300° C., and evacuated to 27-28 inches mercury for 1 hour to remove moisture from the interior surfaces of the pot. A slow stream of helium was then admitted to purge the pot atmosphere while the pot temperature was raised to 700° C. to melt the magnesium. After all the magnesium had been melted, TiCl4 was added at an initial rate varying from 200 to 225 pounds per hour. The furnace temperature and feed rate were regulated so that the skin temperature of the reduction pot, at the controlling thermocouple, was kept in a range of 690° to 710° C. The byproduct magnesium chloride was tapped from the pot at predetermined intervals to aid in maintaining a smooth reaction.
When reaction was completed, the pot was removed from the furnace and allowed to cool to room temperature under helium pressure. Then the weld was ground off, the pot wheeled into the dryroom, where the reaction mass was bored out on a lathe. When subchlorides of titanium were present, they were removed from the top of the reaction mass by a vacuum cleaner and discarded. Titanium subchlorides are extremely pyrophoric and hygroscopic, and their presence is detrimental to the quality of the titanium product.
Byproduct Removal from Crude Titanium Chips
Lathe borings from the reduction pot were caught in a perforated basket. When the basket became filled with crude titanium chips, it was transferred from the dryroom and loaded quickly into a vacuum retort. The retort was evacuated to 50 to 100 microns absolute pressure and held for 30 minutes at room temperature; then it was tested for leaks; all leaks were stopped before the retort was released for the next step.
The retort next was charged to a preheat furnace set at 205° C. and kept there for 2 hours under vacuum. Then the retort was pressurized with helium (or argon) and transferred to the regular vacuum distillation furnace tor distillation.
During the preliminary vacuum and inert gas-purge treatment, some forms of hydrated MgCl2 (mainly MgCl2·6H2O) were dehydrated; the gases resulting from this decomposition were purged from the retort. The temperature of the vacuum furnace was raised to 938° C. and held at that temperature for 32 to 36 hours, depending on the size of the charge in the retort. The magnesium and magnesium chloride distilled from the crude titanium chips and condensed on baffles in the water-cooled zone of the retort.
When the vacuum distillation cycle was finished, the retort was removed from the furnace and allowed to cool to room temperature in an inert gas atmosphere. A 24-hour cooling period was allowed for the 230-pound (approx.) batch of metal. The basket of sponge titanium was then removed from the retort, and a 6-hour air-conditioning period elapsed before the titanium was chipped from the basket. The conditioning period allowed any titanium of a pyrophoric nature to oxidize slowly, thus minimizing any tendency to ignite. Finally the titanium was crushed to minus-½-inch size and stored in air-tight drums.
Summary of Operation
No attempt was made to operate the plant continuously, except in isolated instances where test procedures required more than regularly scheduled 10-day periods. Since the primary purpose of the project was to develop techniques that would produce higher quality sponge, rather than to produce a large quantity of titanium, the equipment was never operated at capacity.
Production from the titanium pilot plant during the 16-month period (June 1955 to September 1956) is summarized in table 2.
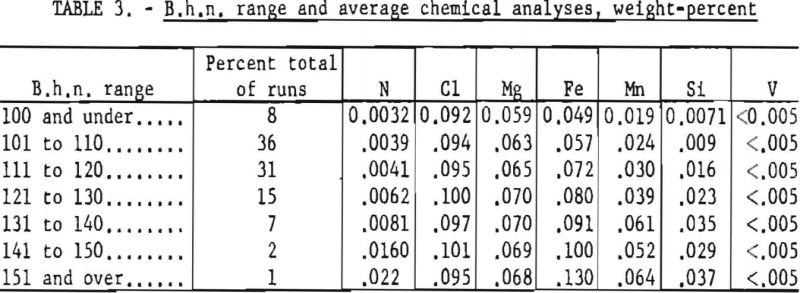
Table 3 lists the B.h.n. range and corresponding average chemical analyses of the sponge metal produced.
For purposes of comparison, results of the cooperative work with the Wah Chang Corp. are included in table 4 with results of previous operation of the Bureau plant during a similar time interval (June 1953 through September 1954). Major improvement in the results of this investigation over that of the earlier work under contract with the Defense Materials Procurement Agency, through GSA, resulted principally from modification of processing techniques, which were based on evaluation of the previous work.
Sources and Effects of Impurities
Titanium metal reacts readily with oxygen, nitrogen, and metallic impurities to form stable compounds which affect the physical properties of the metal. These impurities are difficult to separate from titanium without resorting to chemical or electrorefining techniques. Production of low-hardness titanium consists essentially of making a metal with a minimum of contaminants.
Preliminary efforts were directed toward preparation of very pure feed materials, because any impurities in the feed would ultimately be gettered by the titanium metal. After feed materials of maximum practical purity had been made, final efforts were devoted to minimizing contamination during processing.
A very pure TiCl4 was prepared using existing purifying equipment and incorporating a degassing technique. It did not seem economically feasible to purify magnesium beyond the usual pickling treatment, which removes the surface oxide film. Three separate commercial grades of magnesium were tested; the amount of impurities, however, was reasonably constant for each grade used.
A serious source of contamination in the Kroll batch-type process was exposure of the processing vessels and crude titanium sponge to the atmosphere. Therefore, it was important for all traces of air and moisture to be removed from these processing vessels before the next batch of feed materials was introduced. Air and moisture were removed by a combined vacuum and inert gas treatment. Atmospheric contamination due to leaks which developed during processing, was minimized by careful and alert operators.
Although free gases in the atmosphere contributed to contamination of the titanium sponge, moisture contamination was the more serious effect of exposure. Analyses for nitrogen and oxygen in numerous titanium samples confirm this belief.
Where air leaks occurred, the increase in nitrogen content was much greater than that of oxygen. In the titanium sponge produced by planned methods, however, actual nitrogen content was in the range of 10 to 40 p.p.m. and oxygen 500 to 1,700 p.p.m. Experiments showed that most of the oxygen came from sources other than free gas in the atmosphere; for example, moisture pickup by magnesium chloride, subchlorldes of titanium, titanium tetrachloride, and magnesium oxide.
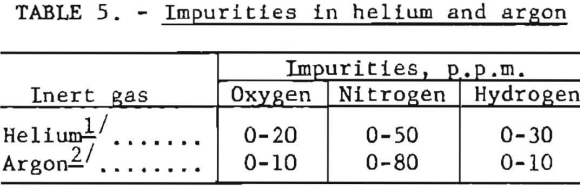
Although helium and argon were used extensively throughout, they may be discounted as an appreciable source of oxygen and nitrogen contamination. Typical analyses of impurities found in these gases are listed in table 5.
Discussion at the end of this report suggests other means by which titanium may be contaminated. The magnitude of the contribution by these individual variables on the quality of titanium metal could not be determined precisely. Techniques applied to check contamination appeared to minimize quality variations between batches of metal produced.
Removal of Atmospheric Contaminants from TiCl4
Theoretical Aspects of Degassing
Since compressed air was used extensively in transfering crude TiCl4 from the producer to purification plant, there existed a strong possibility that some air would remain dissolved In the liquid. To remove the dissolved air and other gaseous impurities present in the crude TiCl4, a degassing technique was devised using helium (or argon) as the scrubbing agent.
Theories on degassing of liquids are discussed in chemical-engineering textbooks.
The degree of solubility of gases in liquids depends on the pressure, temperature, and nature of the gas and solvent. When air, oxygen, nitrogen, and carbon dioxide are dissolved in TiCl4, it is assumed that Henry’s Law applies, and the actual concentration of gas dissolved in liquid is proportional to the partial pressure of gas in the gaseous phase at equilibrium adjacent to the liquid. Air can be released from solution by reducing the partial pressure of air in the gaseous phase and by raising the temperature, thus reducing the solubility of air in the solution.
Reducing the partial pressure of a gas can be done by evacuating the gas space or by diluting the gas above the solution with an inert gas. Evacuation which requires high-vacuum techniques, is not suitable because of the volatility of TiCl4 and the corrosive nature of its vapors. Dilution by an inert gas however, is easily accomplished. By introducing inert gas at the bottom of a tank of TICl4 and allowing the gas to bubble up through the liquid, dissolved gases are scrubbed out of the liquid.
The scrubbing mechanism may be explained by example: Consider that a small bubble of oxygen-free helium gas is present at the bottom of a column of TiCl4 which contains dissolved oxygen. As the bubble rises to the top of the column, some of the dissolved oxygen will diffuse through the interface into the bubble. Diffusion of oxygen will continue until equilibrium of the partial pressure of oxygen in the small bubble of helium is proportional to the amount of dissolved oxygen present in the solution. The bubble of oxygen-free helium gas is as effective as a bubble of vacuum, since scrubbing the gas from the solution depends only on the partial pressure of the solute in the gas phase.
An increase in temperature will decrease the solubility of most dissolved gases. In many instances it is only necessary to heat a solution to expel the dissolved gases; however, the high volatility of TiCl4 at elevated temperatures limits degassing by this technique.
Degassing Method
The sulfiding vessel was conveniently used for degassing the TiCl4. Helium (or argon) was introduced through the same line used for feeding the hydrogen sulfide gas. Agitator blades served to break inert gas into the small bubbles essential for efficient scrubbing.
Raising Sulfiding Temperature
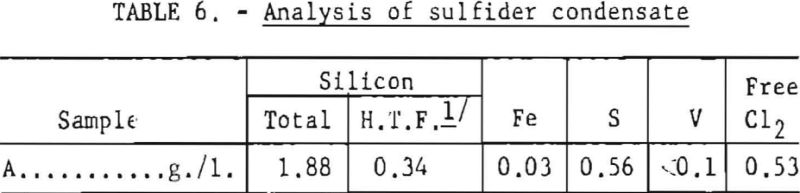
As discussed, raising the temperature oi TiCl4 could decrease the solubility of a dissolved gas and effect its release from solution. This simple operation was tried first to determine whether the hardness of titanium sponge could be thus improved. Accordingly, the sulfiding temperature was raised from 90° to 115° C. The amount of condensate collected at that temperature was approximately 0.1 percent of the weight of a batch (4,300 pounds) of TiCl4. A typical analysis of sulfider condensate is given in table 6.
To determine the effect of higher sulfiding temperature on the final hardness of titanium sponge, 12 test runs were made from purified TiCl4 sulfided at 115° C. Of the 32 runs made from purified TiCl4 sulfided at 90° C., 16 runs were conducted before and 16 after the 12 special runs. In all cases the many variables inherent to the process were held to a practical minimum. The average B.h.n. results of the test runs are given in table 7.
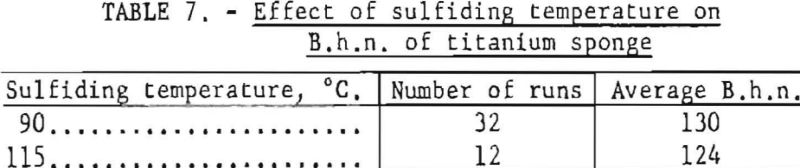
Results indicate that sulfiding HCl4 at the higher temperature of 115° C. Improved the quality of the metal by an average of 6 B.h.n points. The improved quality was probably a result of partial degassing of TiCl4 by a higher sulflding temperature aided by the scrubbing action of hydrogen sulfide on the dissolved gases.
The higher sulfiding temperature also caused the reduction of iron in TiCl4 from a range of 0.03 to 0.05 grams per liter to a consistent value of less than 0.02 grams per liter. Special analytical techniques used by a private laboratory showed an iron content of less than 10 p.p.m. in a representative sample of purified TiCl4.
Degassing TiCl4 With Helium or Argon
Scrubbing the crude TiCl4 with helium before the addition of hydrogen sulfide helped to remove some of the common dissolved gas impurities, such as chlorine, hydrochloric acid, phosgene, and dissolved air. This step minimized unfavorable side reactions with hydrogen sulfide. Scrubbing TiC14 with helium after addition of hydrogen sulfide insured additional removal of the remaining undissolved gases, particularly unreacted hydrogen sulfide gas.
The choice between helium and argon was that of cost, since little difference exists in effectiveness as a degassing agent. Helium was used most because it cost less.
Results of Degassing Tests
To test the combined effect of higher sulfiding temperature and helium scrubbing on the hardness of titanium, 35 controlled tests were made. These test results are listed in table 8; for comparison, the results listed in table 7 are included.
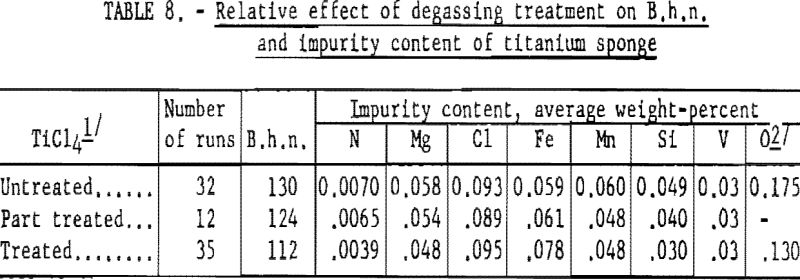
These results indicate that degassing TiCl4 improves the hardness of titanium metal significantly—more so than higher sulfiding temperature.
Instruments for Analyzing Purity of TiCl4
Analytical techniques for most metallic impurities in TiCl4 have been established however, little work has been published on determination of the nonmetalllc impurities present.
Oxygen is one of the contaminants most detrimental to the quality of titanium sponge. For precise evaluation of variations in metal hardness, it was necessary to develop an analytical procedure to determine the amount of dissolved oxygen in TiCl4. The standard Beckman oxygen analyzer was adapted for this purpose. By using the modified instrument and a new procedure it is possible to measure the amount of oxygen dissolved In TiCl4 before and after a scrubbing operation.
Results listed in table 9 were obtained by applying the modified oxygen analyzer to HCl4 saturated with air at a pressure of 1 atmosphere.
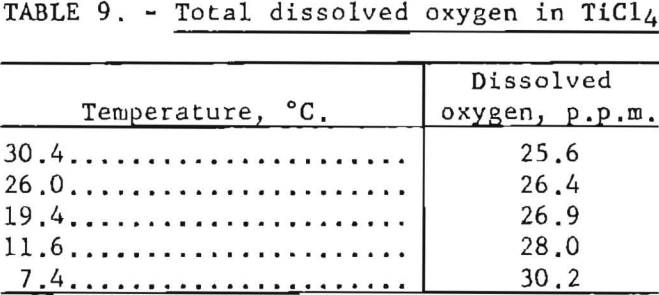
Efficiency of helium as the scrubbing gas was high; passage of a volume of gas equal to the liquid volume eliminated about 99 percent of the dissolved oxygen.
The residual oxygen content in TiCl4 after a degassing treatment using plant equipment was in the range of 0.10 to 0.24 p.p.m. The oxygen content in titanium sponge resulting from 0.24 p.p.m. dissolved oxygen in TiCl4 is 0.96 times 10 -4 percent. Such a quantity would have a negligible effect on the hardness of titanium.
Other impurities found in TiCl4, such as hydrochloric acid, carbonyl chloride, chloroacetyl chlorides, carbon dioxide, free chlorine, vanadium oxytrichloride, and hydrolysis products, have been determined by infrared absorption spectroscopy. Johannesen and coauthors found characteristic infrared absorption peaks of these substances in the spectral region of 3.53 to 8.45 microns.
Work with an infrared spectrophotometer was limited because the apparatus was available only near the end of the cooperative agreement. Some results showed that carbonyl chloride occurred in less than 5 p.p.m., and only traces of carbon dioxide and hydrochloric acid were present in purified TiCl4.
Prevention of Atmospheric Contaminants in Reduction Operation
The reduction procedures used were similar to those described In a recent Bureau report. Additional precautionary measures, which reduced atmospheric contamination of titanium during the reduction operation, are discussed below.
In previous plant operations, the pressure buildup in reduction pots was released through a valve and open pipe directly to the atmosphere. This procedure did not exclude possible diffusion of air back into the pot. For the cooperative experiment, two oil seal bubblers were installed on the vent line, as a preventive measure, and the pressure was vented from the pot to the atmosphere through these bubblers.
In tapping to remove the magnesium chloride byproduct, the previous method of obtaining maximum removal of the molten layer of salt at the bottom of the pot was by tapping until helium was released. Even though a strong flow of helium was escaping through the tapping spout, air diffused back Into the pot, causing nitrogen contamination of the titanium sponge. To prevent this, a molten layer of magnesium chloride was always left in the bottom of the pot to seal the tapping spout.
These practices resulted in minimum variation between batches in nitrogen content and hardness of metal.
Removal of Atmospheric Contaminants in Vacuum Distillation Operation
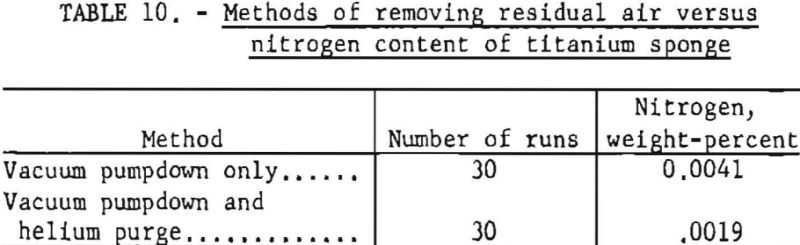
Vacuum treatment alone would require an ultrahigh vacuum to remove practically all residual air from a retort before Ti is subjected to high temperature conditions. To eliminate this requirement a combination vacuum and helium purge was tried, and the inert gases were used to displace the residual air. Sixty runs were made; thirty were conducted by evacuation only; and the other thirty were treated in the manner described previously in this report. Degassed TiCl4 was used for all tests.
Table 10 compares the average residual nitrogen content in titanium sponge with each treatment method.
A further precaution was to check the retort with a charge of crude titanium chips before heating to find any minute leaks in pipe connections and vacuum gasket seals. A special leak detector was used for this purpose. A minute leak could develop into a serious leak at elevated temperatures, and a complete batch of metal would be ruined.
Tests indicated that a combined technique of retort evacuation and helium purge at room temperature plus leak detection removed most of the atmospheric contamination during vacuum distillation.
Minimizing Moisture Contamination in Process
After optimum conditions for the removal of atmospheric contaminants were established, it was found that variations in the B.h.n. of titanium sponge were influenced primarily by oxygen content. Figure 3 shows the effect of
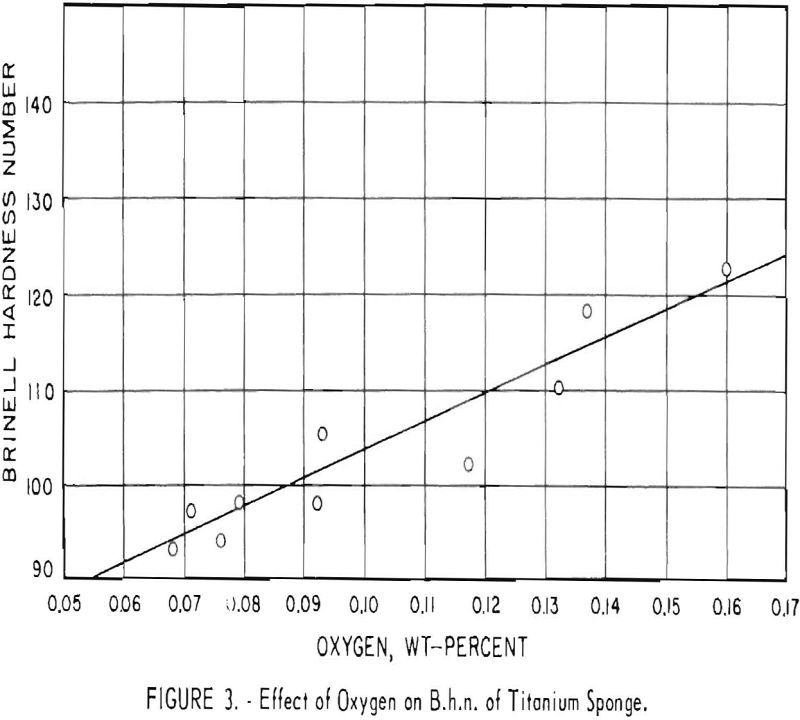
oxygen content on hardness of the titanium sponge obtained in this operation. Although some oxygen is contributed by HCl4 and oxides of magnesium, most of the oxygen in titanium is derived from contamination by moisture.
Moisture enters the process with the raw material TiCl4 and by moisture absorption of anhydrous magnesium chloride, which is formed as a byproduct.
Moisture Contamination in TiCl4 Purification System
Dehydration of Air for Pumping Crude HCl4
The purification system required the use of compressed air to aid in transferring crude TiCl4 from the storage tank to the sulfiding vessel. By allowing pressurized air to bubble through a TiCl4 trap before it was used as a pumping medium, moisture and oil were effectively eliminated; this prevented unnecessary contamination of the raw material.
Effect of Moisture on B.h.n. of Titanium Sponge
Moisture contamination plays a subtle but devastating role in the hardness of titanium sponge. Its presence in TiCl4 escapes visible detection, because the hydrolysis products formed with small amounts of water can exist in solution or as colloidal dispersion.
An excellent example of the effects of water contamination on TiCl4 was experienced when a water-cooled vapor condenser on the fractionating column developed leaks. Increased hardness of titanium was readily evident, but the cause for it was not discovered until the condenser finally failed. Study of the hardness trend showed that water had been leaking into the system for approximately 2 months before failure. Examination of points of failure disclosed that leaks started with pin-size openings on welded joints. These openings enlarged gradually, owing to dissolution of the metal by hydrochloric acid, which was formed from the reaction between TiCl4 and water. Hardness data show further that the detrimental effects of water contamination in the system diminished slowly; hydrolysis products were distributed throughout the entire purification system, and time was required to remove them from the system.
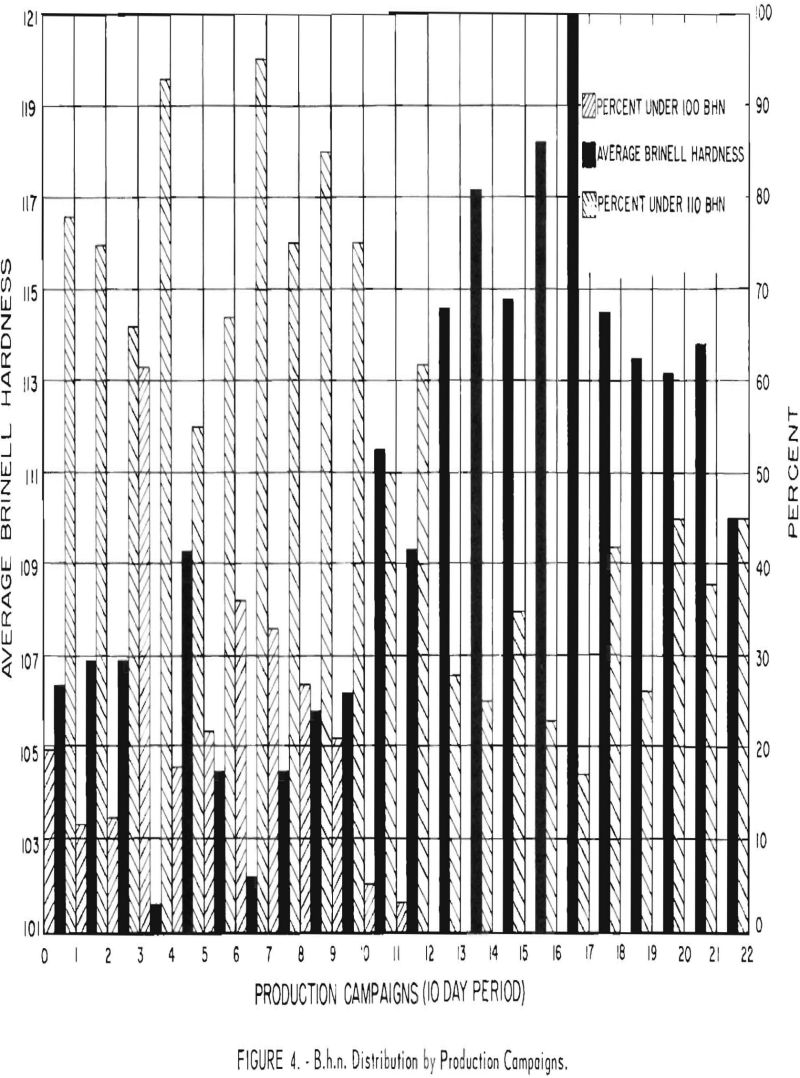
Figure 4 shows the B.h.n. distribution and average B.h.n. for the experimental programs (10-day operating period) before, during, and after water leakage. Batches made during an experiment are classified in two categories: (1) 100-110 B.h.n. and (2) below 100 B.h.n. Water leakage apparently started during test 11. The number of runs below 100 B.h.n. decreased, and the average B.h.n. increased rapidly. During test 14 the condenser ruptured, and during test 17 another leak was found In the replacement condenser. Test 23 was the last.
Safety Hazards
Water contamination of TiCl4 is discussed not only to show its detrimental effect on the hardness of titanium but also to point out a potentially dangerous hazard to safety. The reaction between TiCl4 and water occurs with the generation of pressure and evolution of heat. When conditions are favorable, the reaction can occur with explosive violence. Such a mishap could have occurred when the fractionating column ruptured had it not been for the quick action of the operator who vented the pressure from the system.
Determination of Moisture Contamination in TiCl4
In consideration of the safety hazards and the effect on product quality, a rapid but sensitive method for detecting water contamination of TiCl4 is needed. On the basis of work performed by the National Bureau of Standards for the determination of Impurities in TiCl4 by infrared spectroscopy, the following approach to the solution of the problem is proposed.
The reaction between small amounts of water and TiCl4 yields a hydrolysis product and hydrochloric acid. Both products have been positively identified in the infrared absorption spectrum for TiCl4. Furthermore, infrared techniques are sensitive to small changes in the amount of hydrolysis product in TiCl4. In practice, the initial level and maximum tolerable limit for hydrolysis product and HCl in crude and purified TiCl4 should be established.
Routine checks of TiCl4 by infrared spectrographic analysis could become a part of the procedure. The purification system would have sampling outlets at various points, with samples being taken and analyzed immediately after a showing of excess hydrolysis in the routine sample. In this manner, the location of leakage could be determined and corrected quickly, thus averting possible serious consequences.
As an added precaution, water-cooled vapor condensers in the TiCl4 purification unit should be constructed of a seamless stainless steel in preference to welded mild steel.
Moisture Contamination in Dryroom
Exposure of the reduction pot and its contents in the dryroom affected metal quality in both reduction and vacuum distillation operations. Moisture is absorbed by the anhydrous magnesium chloride in the layer of reaction mass remaining in the reduction pot. This moisture, if not eliminated, would release oxygen to the titanium formed in the succeeding run. The magnesium chloride associated with the raw titanium chips also absorbs moisture but to a much greater extent. When this hydrated material is heated during the vacuum distillation operation, part of the oxygen in the absorbed water is gettered by the titanium chips, resulting in embrittlement of the metal.
The following discussion shows how the quality of titanium was improved by eliminating some of the absorbed moisture.
Theoretical Aspects in Dehydration of Magnesium Chloride
The techniques employed for dehydration of the hydrates of magnesium chloride are based on experimental work of Smith and Veasey and thermodynamic calculations of Kelley. Within limits of errors in calculation and experimentation, their data seem to be in reasonable agreement.
When magnesium chloride hexahydrate is subjected to dehydration, a tetrahydrate, a dihydrate, and a monohydrate are formed successively. The four stages of dehydration are represented by the following equations:
MgCl2·6H2O = MgCl2·4H2O + 2H2O……………………………………………….(1)
MgCl2·4H2O = MgCl2·2H2O + 2H2O……………………………………………….(2a)
MgCl2·4H2O = MgOHCl2 + HCl + 3H2O………………………………………….(2b)
MgCl2·2H2O = MgCl2·H2O + H2O…………………………………………………..(3a)
MgCl2·2H2O = MgOHCl + HCl + H2O……………………………………………..(3b)
MgCl2·H2O = MgCl2 + H2O……………………………………………………………(4a)
MgCl2·H2O = MgOHCl + HCl…………………………………………………………(4b)
The process of dehydration is represented by (a) equations, and that of decomposition by (b) equations. Stage-1 dehydration may be readily accomplished without decomposition. In each succeeding stage, the equilibrium partial vapor pressures of water and hydrochloric acid permit calculations of the relative amount of dehydration and decomposition.
Pertinent data on equilibrium vapor pressure for each stage, according to Kelley, are listed in tables 11 through 15.
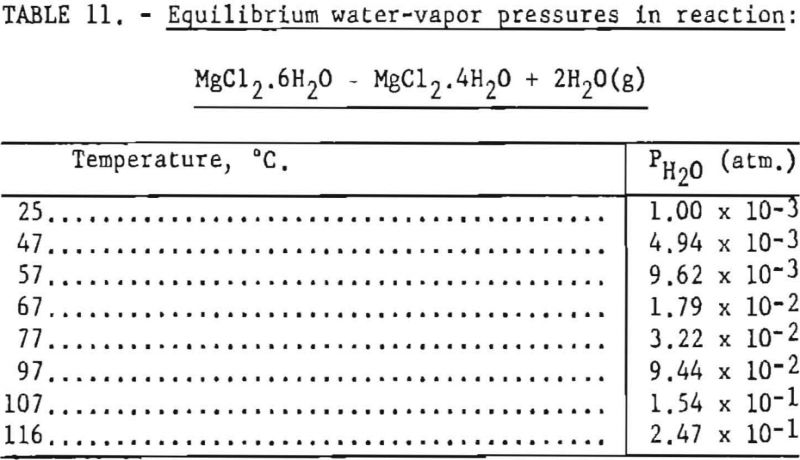
Dehydration of the hexahydrate occurs over the range of temperatures listed in table 11, and the rate of dehydration would be expected to increase as vapor pressures increase at the higher temperatures. It appears that 97° C is the lowest practical dehydration temperature based on easy attainability of associated low pressures. The maximum temperature is limited by partial fusion, which occurs above 116.9° C.
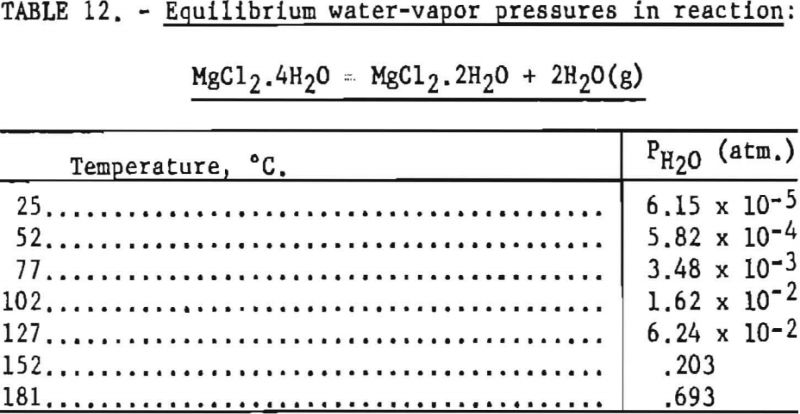
In this step the lowest practical dehydration temperature is above 127° C and the upper temperature is limited to 181.7° C., if partial fusion is to be avoided.
According to data from Smith and Veasey, the partial pressure of hydrochloric acid for the tetrahydrate is very small a maximum of about 4.6 mm. at 180° C. Therefore, for stage 2 the reaction is predominantly that of dehydration.
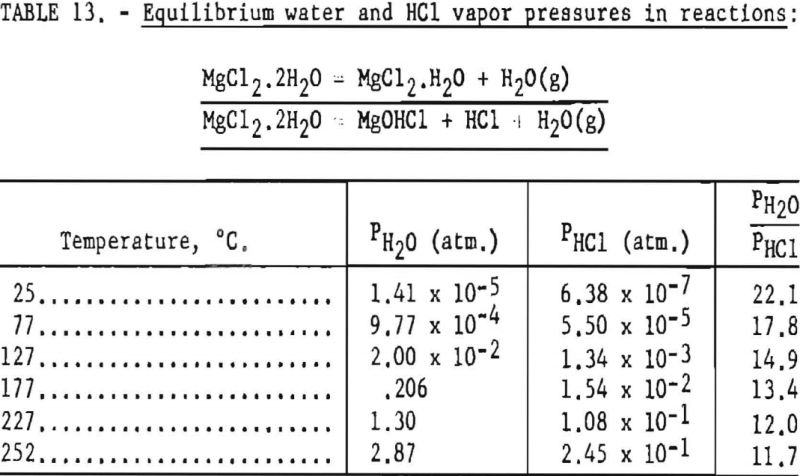
In the range of temperatures listed, data for the dihydrate indicate that the dehydration reaction predominates. For example, at 177° C., the estimated composition of the end product would be 93 mole-percent MgCl2·H2O and 7 mole- percent MgCHCl in the absence of HCl partial pressure.
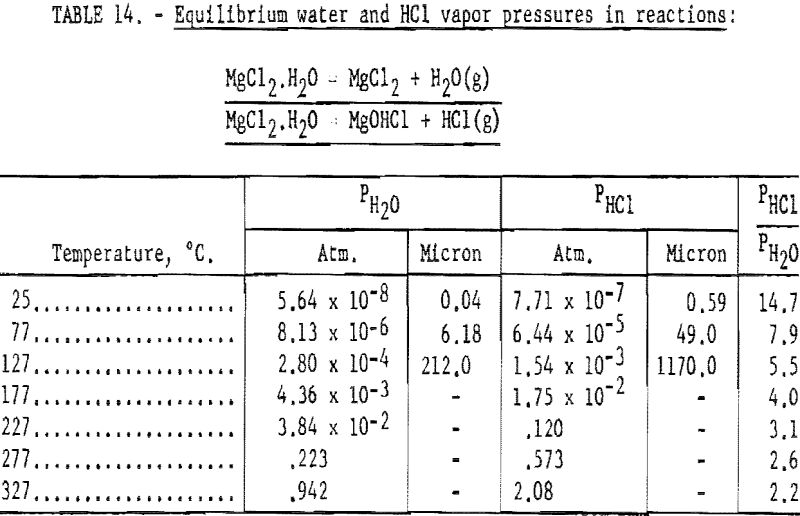
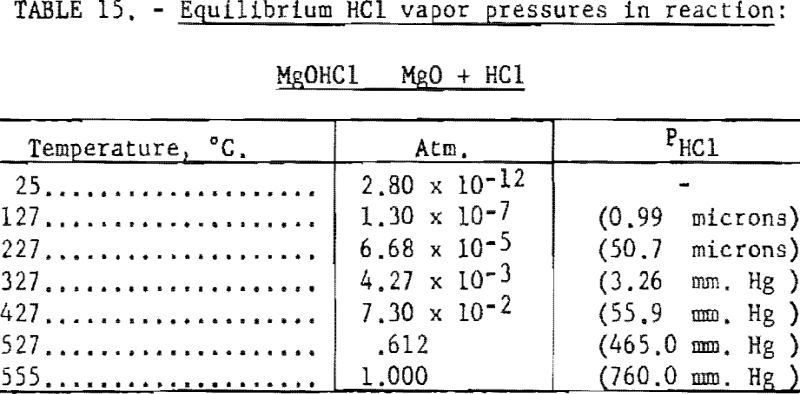
It would appear feasible (see tables 11 through 15) to effect partial dehydration by application of heat to the hexa-, tetra-, and dihydrates of MgCl2 without appreciable decomposition. Drying of the monohydrate in the absence of HC1 atmosphere results primarily in decompos ition, imposing a limitation on the benefits to be derived from the dehydration treatment (see table 14).
The vapor pressure data in tables 14 and 15 indicate that when the vacuum in the distillation retort reaches the range of 50 to 60 microns, the monohydrate will start to decompose and dehydrate at a temperature of 77° C. At this temperature, the partial pressures of water and HCl are about 6.18 and 49.0 microns, respectively, or a total of 55.18 microns. The ratio of PHCl/PH2O is 7.9, which indicates that about 89 percent MgCl2·H2O decomposes into MgOHCl + HCl. Decomposition seems to lessen at higher temperatures, and at 277° C., decomposition of MgCl2·H2O is about 72 percent.
The next step in decomposition involves the reaction MgOHCl MgO + HCl. This reaction will proceed at 50.7 microns pressure at 227° C. (See table 15.) The pressure increases to 760 mm, Hg as the temperature rises to 555° C.
Oxygen Contribution of Magnesium Oxide
The MgO formed during distillation is another source of the oxygen con-tributed to titanium sponge. This oxygen contribution can be reduced by
- carrying out the boring and retort-loading operations in the dryroom under the driest possible conditions to minimize moisture absorption by MgCl2, and
- carrying out dehydration of MgCl2 hydrates at temperatures most favorable to the elimination of moisture in the range 200° to 300° C. at moderate vacuum.
Dehydration Techniques in Reduction Operation
Reduction-pot dehydration operation was simple. Evacuation started immediately after the pot was charged to the reduction furnace. The temperature controller was set at 300° C., and the pot was heated for 1 hour under a vacuum of 27 to 28 inches Hg. When this treatment was completed the pot was raised to the reduction temperature while a constant helium purge was maintained.
Dehydration Techniques in Vacuum Distillation Operation
The dehydration technique used in vacuum distillation involved two steps: (1) Drying moisture from the magnesium chloride imbedded in the wall of the retort, and (2) dehydration of hydrates of magnesium chloride in the basket of crude sponge chips charged to the retort. Before the basket of crude chips was loaded into a retort, the empty retort was heated under vacuum for 1 hour at a temperature of 205° C. and then loaded and purged of all atmospheric gases. (Purging operation was described earlier in the report.) The retort was then charged to a furnace used only for the dehydration treatment. It was heated for 2 hours under vacuum at 205° C., and backfilled with helium (or argon). Finally, the retort was transferred to a furnace for the vacuum distillation cycle.
When the retort was heated to higher temperatures after dehydration, there was usually an increase in pressure from 150 microns to a range of 500 to 800 microns. Sometimes higher pressures were obtained from batches with abnormally high moisture content, Higher pressure indicated decomposition of MgOHCl into MgO + HCl. In runs containing higher moisture content, HCl vapor was readily detected in vacuum pump exhaust gases.
Results of Dehydration Tests
To determine the beneficial effects ot the dehydration treatment, a series of runs was conducted under three conditions. Degassed TiCl4 was used in all test runs. The results of these tests are summarized in table 16.
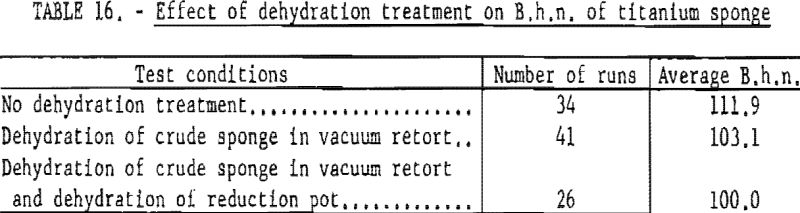
Dehydration of crude sponge in the vacuum retort lowered the hardness of titanium about 9 points B.h.n.; additional dehydration of the reduction pot lowered the B.h.n. another 3 points.
Correlation Studies on Moisture and B.h.n.
Correlation studies show a definite relationship between humidity conditions and the Brinell hardness of titanium sponge. Figure 5 shows a plot of the effect of outside humidity on the dewpoint of the dryroom. Dewpoint increased as humidity increased. The capacity of the air-drying unit and its operation are described in a previous report.
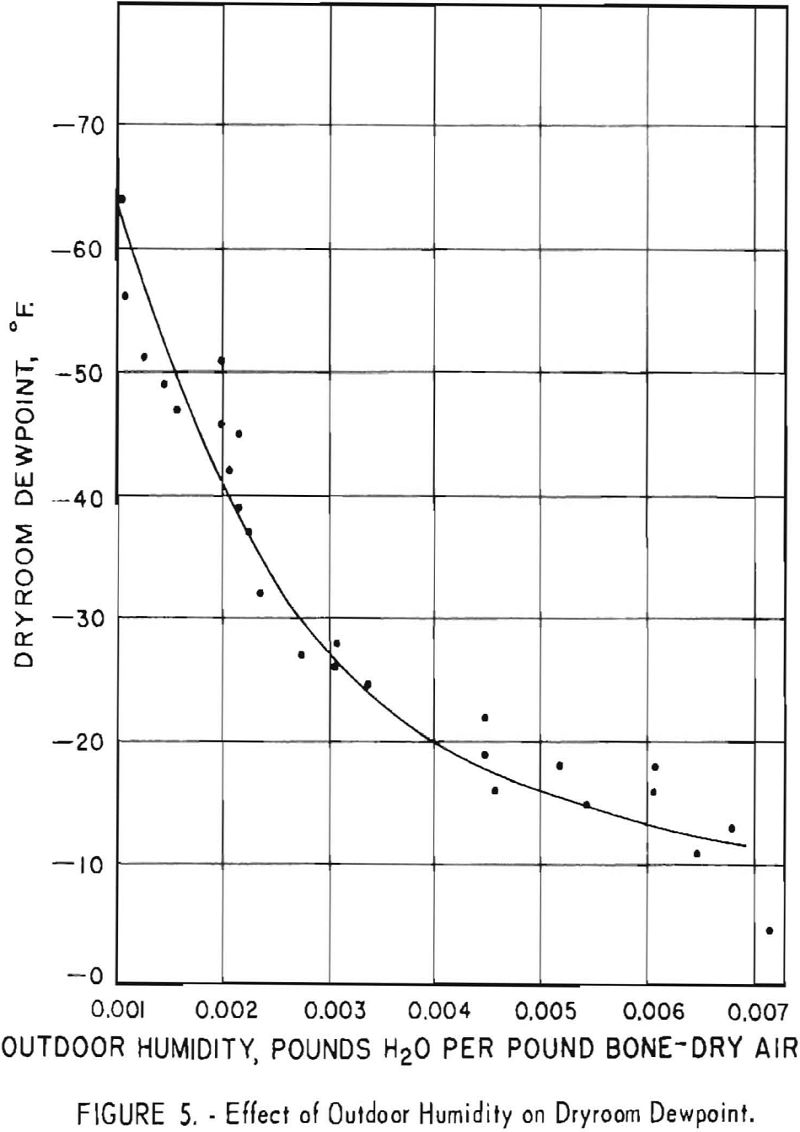
Figure 6 is a graph showing the actual water content per cubic foot of air at various dewpoints. The water content drops sharply from a dew- point of 0° F. to -40° F.
The 139 batches that had a hardness of 110 B.h.n. or less, which were handled in the dryroom when the dewpoint range was -40° to -65.5° F., represent 40.8 percent of the 341 batches produced. Insofar as the hardness of the sponge is affected by oxygen resulting from moisture contamination during dryroom operations, indications are that the dewpoint of the dryroom should be maintained at a minimum level of -40°
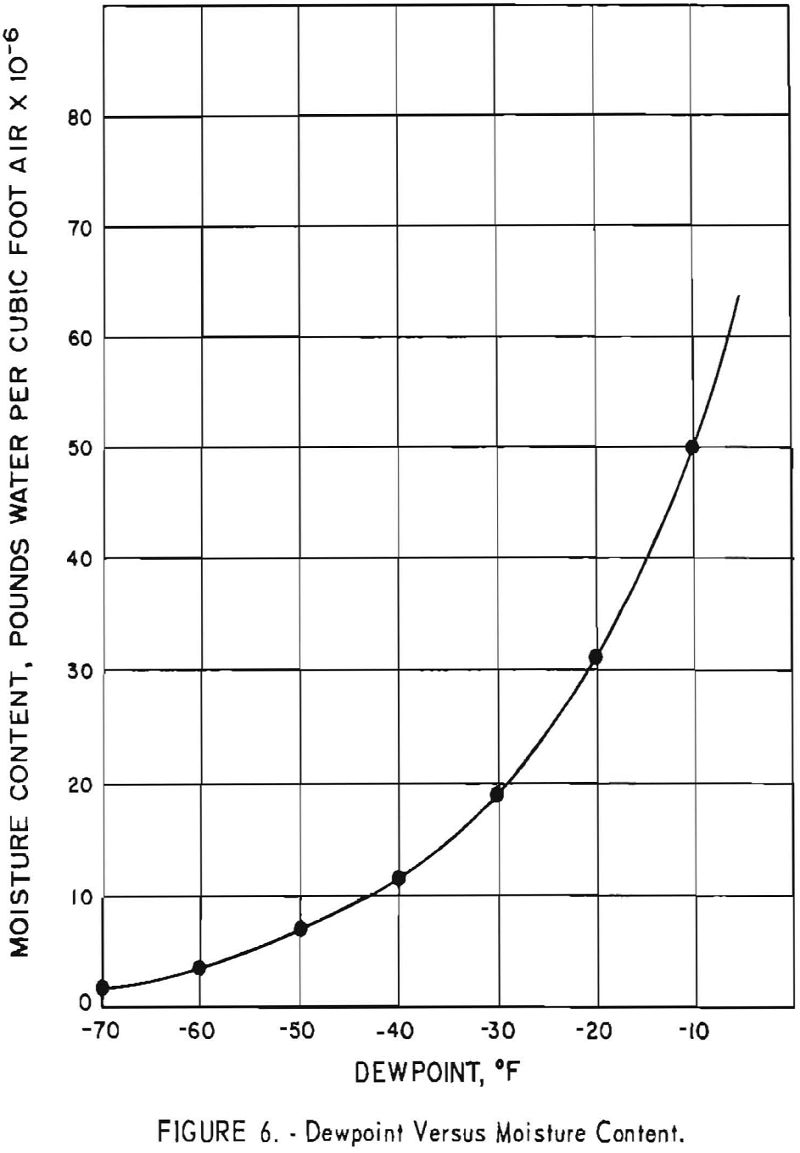
to obtain low-hardness titanium consistently. An air-drying unit capable of maintaining a steady dryroom-humidity condition could help minimize quality inconsistencies between batches of metal.
Effect of Lower Chlorides on B.h.n.
Lower chlorides of titanium, mostly present in a divalent form, had a deleterious effect on the B.h.n. of titanium sponge. The mixture of titanium dichloride and trichloride was usually formed at the end of a reduction operation when a deficiency of magnesium existed. Correlation studies show that in batches of titanium with hardness of less than 102 B.h.n., lower chlorides were always absent. However, in batches of metal with a hardness greater than 120 B.h.n., lower chlorides were present in 69.2 percent of the batches.
The reaction between lower chlorides and moisture appeared to proceed at a rapid rate, as evidenced by evolution of heat and smoke. The odor of hydrochloric acid gas usually was present, and an oxychloride product was believed to be formed. This substance would be an additional source of oxygen contamination of titanium sponge.
Formation of lower chlorides can be minimized by careful control of TiCl4 feed rates, thus avoiding force-feeding, especially near the end of a reduction operation.
Control of Other Impurities in Titanium Sponge
Removal of Vanadium and Iron Compounds in TiCl4
During the distillation of TiCl4 from precipitated residues, minute solid impurities were carried over to the purified TiCl4 storage tanks. These solid matters settled in the bottom of the storage tanks, and were constantly flushed through transfer lines into the reduction pot. To prevent the carryover of such impurities to the metal being formed, the TiCl4 was forced through steel pipes packed with glass wool. The dark green residue trapped In the glass wool was analyzed qualitatively. (See table 17.)
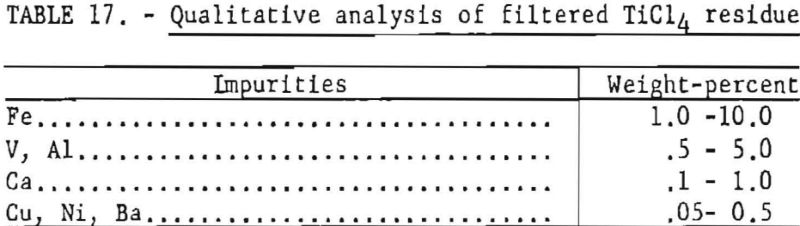
Although removal of solid matter in TiCl4 did not show any appreciable effect on hardness of the metal, any reduction in the impurity level of the TiCl4 is helpful in maintaining consistency of quality in the material produced from it.
Impurities Introduced by Magnesium
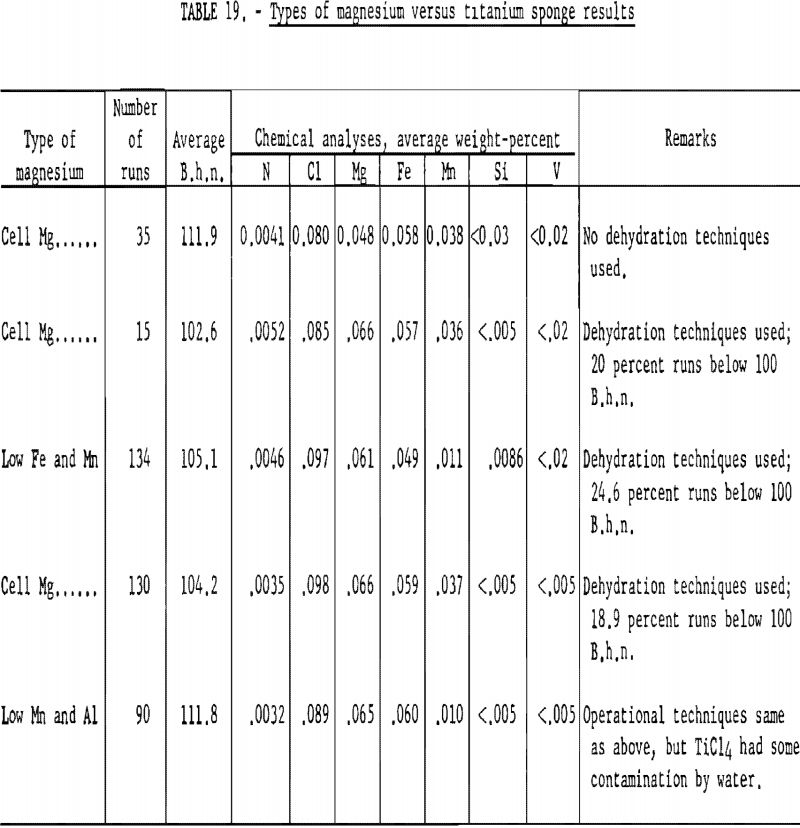
To investigate the effect of magnesium on the hardness of titanium, three grades of magnesium were used. Analytical results of the various grades as determined by the Bureau of Mines laboratory are listed in table 18.
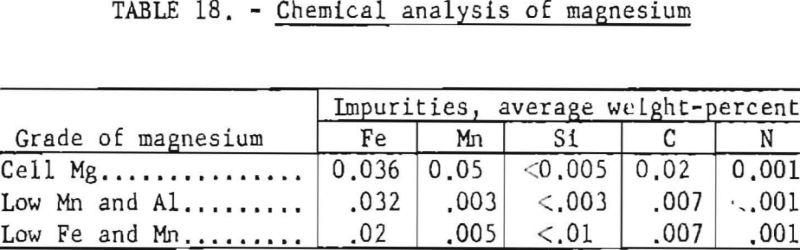
The average B.h.n. and chemical analyses of titanium sponge reduced by the three grades of magnesium arc given in table 19. The order of listing corresponds to the sequence in which the different grades were used in the operation. The variables are listed under “Remarks.”
Based on the results obtained, the following conclusion may be stated:
The different grades of magnesium had no significant bearing on the hardness of titanium sponge produced. Regular cell-grade magnesium yielded metal in the same B.h.n. range as that made from magnesium with low Fe, Mn, and Al content.
Contamination of Titanium Sponge During Vacuum Distillation
In the early stages of distillation the process of vaporization and condensation of magnesium and magnesium chloride occurring within a retort is comparable to the functioning of a diffusion pump. In time, after most of the magnesium and magnesium chloride have been removed, the gettering action of hot titanium causes a vacuum to develop in the retort that exceeds the vacuum capacity of the mechanical pump. This transition usually occurs after 16 to 20 hours at distillation temperature. It was essential to close the valve on the line to the vacuum pump to prevent contamination of the titanium metal in the retort by the backflow of volatile oil vapors. The exact time for closing the valve was determined by measuring the vacuum in the retort and at the pump.
Observance of this practice resulted in the absence of carbon deposits in the condensing zone of the retort and consequently eliminated hydrocarbon contamination of titanium by vacuum pump oil.
Although an oil filter was used on each pump, the filter capacity was inadequate. In the absence of a continuous oil-purifying system, inert gas was bubbled through oil at above-normal operating temperatures, improving both the vacuum and pumping efficiency of the vacuum pump. The helium used in purging a retort also served to purge the oil in the vacuum pump.
