Table of Contents
Improve the strength and other properties of steel alloys.
Approach
Research by the U.S. Bureau of Mines indicates that steels melted and solidified under high nitrogen pressure acquire yield and tensile strengths that are up to four times the strengths of comparable stainless steel alloys without nitrogen. Fatigue strength, creep strength, and other mechanical properties are also improved. Strengthening occurs by two mechanisms: (1) increasing dissolved nitrogen in the lattice and (2) formation of dispersed metal nitride phases.
Nitrogen Alloying
 Nitrogen alloying of a steel is generally done by introducing metal nitrides during melting of the steel. The concentration of nitrogen is limited to its equilibrium solubility at 1 atmosphere. For face-centered-cubic (fee) iron, that solubility is approximately 0.4 weight percent (wt%). For body-centered-cubic (bcc) iron, solubility is 0.05 wt%. This solubility limitation can be overcome by melting the metal in a pressurized vessel.
Nitrogen alloying of a steel is generally done by introducing metal nitrides during melting of the steel. The concentration of nitrogen is limited to its equilibrium solubility at 1 atmosphere. For face-centered-cubic (fee) iron, that solubility is approximately 0.4 weight percent (wt%). For body-centered-cubic (bcc) iron, solubility is 0.05 wt%. This solubility limitation can be overcome by melting the metal in a pressurized vessel.
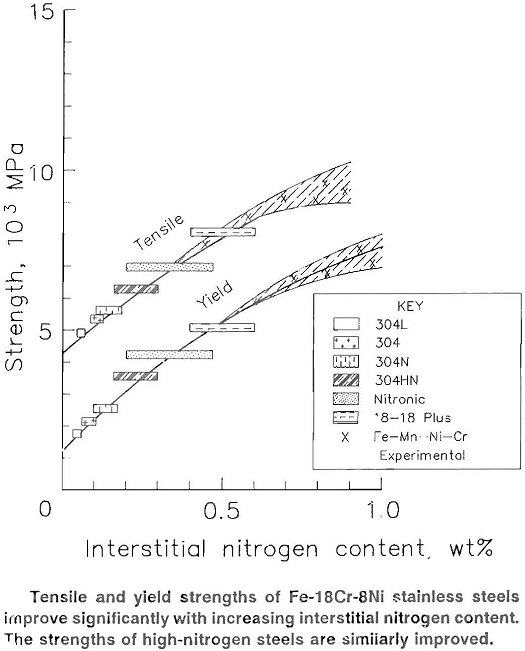
European steelmakers currently have metal-processing systems that can melt and pour tons of steel under nitrogen pressures exceeding 4 megapascals (MPa) (40 atmospheres). They have achieved nitrogen concentrations as high as 1.0 wt% in these (stainless) steels. The tensile strength is approximately twice the tensile strength of similar steels without nitrogen.
Researchers at the Bureau recently melted steels in a hot isostatic-pressure furnace with nitrogen at pressures from 0.1 to 200 MPa. At the higher pressures, Bureau researchers observed the same trends noted by the European steelmakers: higher nitrogen concentrations and higher tensile strengths for both fcc and bcc iron alloys. At 200 MPa, nitrogen concentrations exceeded 4 wt% in fee iron alloys and 1.0 wt% in bcc iron alloys.
The solubility of nitrogen in iron alloys depends on both the crystal structure and the alloy composition. Nitrogen solubility in steel increases with either chromium or manganese concentrations, and it decreases with nickel concentrations. For example, in some steels where manganese replaces nickel as the principal austenitizing alloy component, nitrogen solubility is increased by a factor of 2.
Currently, nitrogen alloying additions are made principally to fee stainless steels. In austenitic fee stainless steels, the concentration of nitrogen in solid solution increases linearly with pressure until it is sufficient to bring about a phase change or to cause a second phase to form.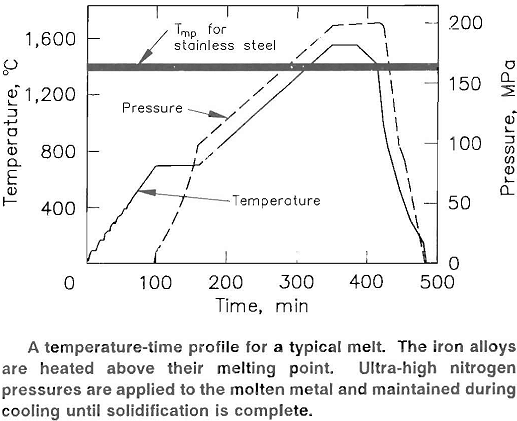
Application of Ultra-High Pressure
For bcc iron alloys, increasing the nitrogen overpressure from 0.1 to 100 MPa and then to 200 MPa increased the nitrogen concentration in the steel from 0.03 to 0.3 wt% and then to 1.2 wt%. For fee iron alloys, the same pressure regimen increased the concentration from 0.03 to 0.3 wt% and then to greater than 4.0 wt%.
Metal charges were heated to 1,650° C and held under pressure for an hour. Then the charges were cooled (under pressure) until the metal solidified. Iron and iron alloys were thus heat- and pressure-treated:
- Pure iron was melted under a nitrogen pressure of 50 MPa. Second-phase iron nitrides, Fe4N and Fe2N, were formed.
- Iron-chromium alloys were melted under a nitrogen pressure of 100 MPa. Chromium nitride (CrN) was formed.
- Three series of alloys—iron-nickel (Fe-Ni), iron-chromium (Fe-Cr), and iron-chromium-nickel (Fe-Cr- Ni)—were melted under varying nitrogen pressures and for different times at temperature. The size and morphology of nitride precipitates depended on the alloy composition and nitrogen pressure; possibly they also depended on time at temperature. In Fe-Cr-Ni alloys, CrN concentrations as high as 25% were observed, yielding a metal nitride composite microstructure.
- Commercial austenitic stainless steels (Fe-18Cr-8Ni) that were melted under nitrogen pressure of 200 MPa also formed CrN. The formation of a high volume of CrN depleted the chromium content of the matrix to below 10 wt%, but an austenitic microstructure was retained.
The workability of the high-nitrogen Fe-Cr-Ni alloys was comparable to that of the same alloys without nitrogen. The alloys were readily worked at conventional hot-working temperatures of 800° to 1,050° C. Although the CrN dendritic microstructures, broke up during hot-working, no dissolution or decomposition was observed. It is postulated that because of the stable, high-nitrogen concentration of the matrix, the CrN phase remains in thermodynamic equilibrium.
As-cast, high-nitrogen Fe-Cr-Ni alloys have high tensile strengths, but are brittle. The hot-worked alloys exhibited not only higher tensile strengths than their as-cast counterparts but also tensile elongations of over 25%. The yield strength of hot-worked Fe-18Cr-8Ni alloys with high nitrogen, compared with that of their source alloys, commercial low-carbon equivalents, was increased from approximately 170 MPa to more than 700 MPa. After hot-working, the alloys were also amenable to cold-working to further increase tensile strengths.
Advantages of Nitrogen Alloying
Nitrogen enters steel as interstitial atoms and forms a solid solution. The nitrogen concentration in steels can be controlled by adjusting both the alloy composition and the nitrogen overpressure. Thermodynamic calculations can be used to estimate the interactions between nitrogen and the alloy elements as nitrogen concentration increases, that is, what type of nitrides will be formed and under what conditions.
Nitrogen and carbon compete for the interstitial sites within the metal lattice. Because of this competition, the presence of carbon decreases the solubility of nitrogen.
However, since nitrogen is more soluble in iron than carbon is before the respective chromium precipitates form, nitrogen alloying is preferred in fcc iron.
The addition of massive quantities of nitrogen to metals, especially to iron alloys, is new. The resulting growth of nitride networks within the molten metal raises the possibility of producing new metal nitride composite materials. With iron-nitrogen alloys, different alloying elements can be combined to form only selected nitrides. Studies currently underway indicate that with the addition of aluminum or silicon to stainless steels, aluminum or silicon nitride, AlN or Si3N4, is preferentially precipitated and the quantity of CrN formed is decreased.
Applicability of Nitrogen-Alloyed Steels
Laboratory heats of nitrogen-alloyed steels have been produced that have significantly higher nitrogen contents than previously obtained. The conditions under which these nitrogen-alloyed steels were prepared are within the temperature and pressure capabilities of many commercial hot isostatic-pressure furnaces designed to sinter ceramic materials. Therefore, facilities exist that could produce commercial quantities of these high-nitrogen alloys.
Today many applications of advanced materials require high tensile strengths, high wear resistance, and excellent corrosion and oxidation resistance. High-nitrogen alloys and precipitated metal nitride composites promise to fill some of these requirements with iron-based materials.
In addition, melting metals under high gas pressures is a new technology that may be applicable to other gas-alloy systems.
Additional Benefits of Nitrogen-Alloyed Steels
Nitrogen addition to austenitic stainless steels (containing about 19 wt% chromium and 9 wt% nickel) has been demonstrated to increase fatigue life and to decrease creep rates for constant applied stress levels. Thus, the operational lifetime for many high-temperature applications, such as steam and nuclear power plants, could be extended by using nitrogen alloying.
Nitrogen addition increases the stability of the austenitic structure. Therefore, alloys with high nitrogen content tend to contain less ferrite or martensite and more austenite. The ferritic and martensite structures have lower toughness and higher fatigue-crack growth rates, especially at low temperatures. Hence, nitrogen alloying increases resistance to both fracture and fatigue-crack growth in metastable austenitic alloys.
The presence of nitrogen leads to the formation of passive oxide films that better shield austenitic alloys from corrosion. Pitting corrosion is decreased, and corrosion resistance to acids is increased. However, all forms of corrosion resistance are still dependent on the presence of sufficient amounts of chromium in the passive films.
The increased resistance to fatigue, creep, and corrosion is expected to lead to the use of nitrogen-alloyed steels in power generation, ship building, chemical and cryogenic processes, and pressure vessels. Currently, these steels are being produced for generator retaining rings. The increased fracture toughness of austenitic steels (compared with ferritic steels such as maraging and Ni-Co steels at the same strength level) could lead to eventual substitution of the austenitic steels for other steels in many applications.
Patent Status
The U.S. Department of the Interior does not plan to apply for a patent on this process.

