Table of Contents
Large quantities of scrap metals are discarded each year by industry and householders; recycling of these materials results in conservation of dwindling domestic resources and helps ease U.S. dependence on imports. Substantial energy savings are also achieved by increased use of recycled material.
In order for these scrap metals to be returned to those operations where they can be recycled effectively, they must first be sorted and segregated into lots that contain similar materials. The first and most critical phase in this operation is the identification of the metal or alloy.
In the routine operation of a commercial scrap yard, identification and segregation is carried out by experienced scrap sorters. The degree of separation the scrap metals receive at the scrap yard depends on the abilities of these sorters to identify the alloys with which they come into contact. This is commonly done by object recognition or by a limited number of physical or chemical tests.
A widening variety of new alloys now is entering the scrap market, making recognition increasingly difficult, even for the experienced sorter. The problem is compounded further by the decreasing number of available, skilled scrap sorters. This skill in recognition can be achieved only through day-to-day, hands-on experience in the scrap yard or plant.
Many of these newer alloys are chemically complex, so recycling of these alloys would be greatly facilitated if they were separated as discrete alloys rather than as a group of alloys with a common base. In fact, separation into discrete alloys is the ideal situation for all recycling operations, as it would enable recycling to be achieved without the expense of refining, diluting, and realloying. This in turn would maximize the financial return to the dealer.
Identification of scrap may be accomplished by object recognition and by considering color, apparent density, magnetic properties, nature of sparks resulting when a metal or alloy is touched to a grinding wheel, chemical spot tests, and by the more time-consuming methods of chemical and spectrographic analysis. Some of the commercially available devices include fluorescent X-ray spectrographic analyzers, portable optical emission devices, and thermoelectric sorters. In addition, a number of techniques used in other fields of testing may have the potential to identify and sort certain metals and alloys.
The purpose of this report is to review both currently available and potential methods of scrap metal identification. Within the various techniques, a number of commercially available instruments are noted. It should be emphasized that these are not intended to be complete lists, but are included to inform the reader of the types of equipment commercially available. Also, reference to specific company or trade names does not imply endorsement by the Bureau of Mines. Similarly, omission of specific company or brand names does not imply disapproval by the Bureau of Mines.
Sorting Strategy
Scrap metals are normally classified into three categories: home scrap, prompt industrial scrap, and obsolete scrap. Home scrap is generated within the melting or processing facility and is recycled back into the melting furnaces. Prompt industrial scrap, which is normally generated within fabricating and manufacturing operations, may be recycled back to the melting and refining facilities if care is taken to keep it segregated and free of contamination. Obsolete scrap (or postconsumer scrap) is old scrap generated at the end of the product life cycle. This material presents the major problem for identification and segregation.
Prior to sorting of scrap, it is necessary to select the groups into which the various materials are to be sorted. There is a very large number of commercial alloys available and, this number is increasing substantially as new alloys come onto the market. This wide variety of alloys makes it a practical impossibility to separate all scrap into distinct alloys. As a result, much scrap is sorted into more broadly based groups. The sorting method used needs to be tailored to enable the required separation with the highest efficiency.
Standard classifications for nonferrous scrap and for iron and steel scrap have been published by the National Association of Recycling Industries (NARI) and by the Institute of Scrap Iron and Steel (ISIS). These designations are made on the basis of the chemical composition and the physical condition of the material. They serve as a good basis for sorting scrap into marketable materials, and cover those items that are traded most frequently. Classifications other than those published by NARI and ISIS are also used.
The following are examples of classifications:
ISIS No. 209:— No. 2 bundles: Old black and galvanized steel sheet scrap, hydraulically compressed to charging box size, and weighing not less than 75 lbs per cubic foot. May not include tin- or lead-coated material or vitreous enameled material.
NARI Honey:—26-yellow brass scrap: Brass castings, rolled brass, rod brass, tubing, and miscellaneous yellow brasses including plated brass. Must be free of manganese bronze, aluminum bronze, unsweated radiators or radiator parts, iron, and excessively dirty and corroded materials.
When deciding on the categories into which a material is to be sorted, the economics of the operation is of major importance. From a dealer point of view, it is best to sort into those categories that will result in the greatest dollar return. This, however, does not always result in separating the most valuable or critical groups from the scrap. Other factors such as insufficient quantities, difficulties in identification and separation may make it economically more viable to downgrade some of the more valuable or critical materials. The more valuable or critical alloys may constitute only a small fraction of the total, which, is separated, would be insufficient for sale. It may, however, be found that in order to meet a given specification, material of higher alloy content must be removed or lower grade material must be added to dilute the effect of the higher alloy. If sufficient quantities of the high alloy material are involved, and if the extra return for separating is warranted, then this material would be removed. If not, other material must be mixed with the batch so that it will meet specifications.
An example of this can be found within the AISI type 300 series stainless steels. The NARI classification Sabot 125 calls for clean 18-8 grade stainless steel clips and solids containing a minimum 7 pct nickel and 16 pct chromium and a maximum of 0.5 pct molybdenum, 0.5 pct copper, 0.045 pct phosphorous, and 0.03 pct sulfur, and otherwise free of harmful contaminants. Within this series, type 316 stainless steel contains 2 to 3 pct molybdenum, and certain less-common grades also have significant amounts of molybdenum. In order to meet the specification, these grades must constitute less than 10 to 15 pct of the total; otherwise, they must be removed or diluted. In this case, the higher molybdenum grades will usually bring a higher price due to the high cost of molybdenum, and it becomes economical to separate the molybdenum grades from the others, provided enough material is available to make up a salable parcel.
The potential reward for separating material into discrete alloys increases as the value of the alloy content increases. In addition, there is a potential penalty of increasing magnitude if contaminating material is present. This is especially true for certain superalloys. These often contain significant quantities of several alloying elements, and elements that may be beneficial to one alloy may be harmful to another. For these alloys, therefore, the incentive exists for separating into discrete, uncontaminated alloys, and sophisticated identification, separation, and cleaning techniques can be justified. For alloys of lower value, this incentive does not exist and simpler methods will suffice.
Preliminary Sorting
The preliminary process for identifying metals involves judgmental decisions on the part of the sorter based on the shape, color, and weight of the material. This is often followed by testing with a small hand-held permanent magnet. These methods are sometimes all that is necessary for adequate segregation. When separating into standard classifications, the physical nature and size of the material as well as its chemical composition are considered.
Object Recognition
Some alloys can be easily identified on the basis of known commercial applications. For example, cocks and faucets usually made of similar alloys (yellow brass) are quickly sorted into a “cocks and faucets” category for resale, also, certain valve bodies are typically made of red brass and can be sorted by object recognition alone. Thus, if the class of alloys from which a particular object is made is known, then the sorting process can be greatly simplified. At best, a particular designation can be assigned to the alloy; at worst, the number of possibilities can be greatly reduced.
Unfortunately, a great deal of experience is required to make effective use of this method. In addition, the amount of experience required increases markedly with the diversity of the operation, as the number of alloys and objects likely to be encountered in a wide-based scrap business is much greater than in a small business. As an example of the use of this method for identification, stainless steel cutlery will be made from AISI types 410, 420, or 440 grades. Similarly, plumbing fittings may be made of red brass, semired brass, or yellow brass. These brass alloys can be distinguished from each other by color and the appearance of their drillings.
Information regarding uses and applications of alloys is not usually presented in this way. More usually, various alloys are listed and their applications noted along with chemical composition, mechanical properties, etc. Information of this sort is readily available in the technical literature (25, 28), from industry groups such as the Copper Development Association, the American Iron and Steel Institute, the Aluminum Association, and from the producers of the various types of alloys. This type of information can be used by the sorter to aid in identification.
A wide range of alloys may be used for certain applications. This is especially true for the more complex alloys and superalloys that are required for high-temperature oxidation and corrosion resistance. Alloys of different composition can have similar applications, and the use of object recognition can serve only to define the material as a superalloy, but not make the closer separations into the specific alloy.
Color
A number of metals and alloys have a characteristic color, thus visual examination of color can be used to make an initial separation. In certain cases, a definite identification can be made on this basis.
When using color to identify metals, it is important that a clean, freshly prepared surface of the base metal be examined in order to eliminate the effects of coatings, corrosion products, dirt, etc. The clean surface can be obtained by filing, grinding, drilling, or shearing. If drillings are used, it should be remembered that the color of the drillings can vary depending on the amount of pressure used to obtain the drillings and on the type of drill and bit used.
The material should be examined in good light but not in direct sunlight. Artificial lights are available that will give the same characteristics as daylight. It is also important, in all cases where identification is made by the color of the break, or by drilling or filing, that the examination take place immediately. Exposure to the environment, even for a relatively short time, may change the color of the metal, making identification more difficult.
A preliminary sorting based on color can be carried out according to table 1.

A close examination of the drillings may allow a separation within the groups shown in table 1. This is especially true for the brasses and bronzes. Examination of the drillings should be performed on the dull side, as the shiny side will reflect the light. This is important when trying to distinguish between alloys where the color difference is only slight. In addition to color, the type of chip should also be noted.
The Institute of Scrap Iron and Steel developed a Copper Base Alloy Selector Kit as an aid to the identification of common brass and bronze alloys in solid form. The kit contains drillings taken from 10 known alloys. To utilize the kit, a ½-in drill is used to take drillings from the metal to be identified. The drillings from the metal are compared with those of the kit; both color and type of chip are compared. Allowances must be made for color changes of the standard drillings owing to oxidation.
In certain cases, the appearance of a fractured surface rather than a cut surface or drillings can be used to make an identification. For example, Muntz metal and admiralty metal are both used for condenser tubes and both have drillings of a golden yellow color. If a fracture surface is examined, however, the Muntz metal break will appear brown, while the admiralty metal break will exhibit a green cast.
Weight
Since metals vary in specific gravity from 1.74 for magnesium up to 21.4 for platinum, an initial separation can be made on this basis as listed in table 2.
Separation into the various groups is made by the sorter by picking up various objects and classifying them into the categories listed in table 2.
As with preliminary sorting by color, it is possible to make separations within the groups by a more accurate density determination. For example, within the light metal group, magnesium, aluminum, and titanium have a specific gravity of 1.74, 2.70, and 4.5 respectively. The addition of alloying elements does not significantly affect these values. Thus, alloys of each of these three metals may be readily distinguished from each other by a simple density determination. A piece of the material is taken and weighed. Its volume is then determined by placing it in a cylinder of water and noting the displacement of water. The density, determined by dividing the weight by the volume, is a reliable value for sorting purposes.
Magnet Testing
Magnet testing involves determining whether or not a material is ferromagnetic, that is, attracts a magnet. This test is made with a permanent magnet that can either be suspended over a convenient location or kept in the sorter’s pocket. The loosely hanging magnet is brought to a vertical surface of the test piece. By testing in this way, any movement of the magnet toward the test surface will be readily detected.
There are three ferromagnetic metals, iron, nickel, and cobalt Among the alloys, the iron-base alloys are most likely to be ferromagnetic, although a few nickel alloys are also magnetic. The 300 series stainless steels are inherently nonmagnetic in their fully annealed condition. However, even small amounts of cold work will cause some of these steels to become substantially magnetic. With increasing cold work, the magnetic properties change markedly, depending on the composition of the metal. Certain iron-containing copper alloys are also slightly magnetic. A summary of magnetic properties is given in table 3.

Spark Testing
Spark testing is based on the property of some metals in the finely divided state to oxidize rapidly when heated. When such materials are ground by a high-speed grinding wheel, the fine particles torn loose are oxidized and raised to an incandescent temperature through the heat of friction on the wheel. Experienced sorters can, by observing the sparks produced in this way, use this method to identify various metals and alloys.
The spark test is conducted by placing the material in contact with a high-speed portable or stationary bench grinder with a surface speed of -7,500 fpm. The specimen should be held so that the sparks fly off horizontally. An 8-in-diam wheel rotating at 3,600 rpm has a surface speed of about 7,500 fpm. The surface speed of the wheel is important, as the faster the wheel, the larger and longer the spark stream and the less pressure is needed. A wheel turning at a surface speed of 3,600 fpm requires considerable pressure to obtain a useful trail of sparks, whereas very little pressure is required at 7,500 fpm.
The type of grinding wheel is important and must be suited to the types of metal being spark tested. For normal carbon and constructional alloys, a Carborundum Aloxite resinold wheel is usually used. For identifying other materials such as tool steels or stainless steels other wheels may be better suited. The grain size of the abrasive in the wheel does not seem to be very important. A 30-grain wheel will give somewhat less spark than a 60-grain wheel if all other factors are equal.
Possible contamination of the spark from particles retained in the wheel from previous tests can be a problem. It is important, therefore, that the wheel be dressed clean before spark testing. It is likewise important that the surface tested should truly represent the bulk material. The specimen must be cleaned with a degreasing solvent, emery cloth, or file to remove dirt, grease, corrosion products, metallic coatings, or any decarburized or carburized layer. In spark testing steels, the heat treatment to which the piece has been subjected also has an effect on the spark and must be taken into account. Hardened steels generally throw a longer spark than annealed steels.
Proficiency in spark testing requires practice in identifying the sparks and in reproducing sparking results, so that a given material will show the same sparks whenever it is tested. To obtain this reproducibility, care should be taken to apply the same amount of pressure over the same sparking area in each test. Only enough pressure to maintain a steady contact between the material and the wheel should be used.
The lighting conditions should be approximately the same for each test if reproducibility of results is to be attained. Spark tests should not be made in bright sunlight or in the dark. Diffused daylight or artificial light that approaches daylight is best. In the descriptions of spark trails, a number of terms are used to describe parts of the trail. These are listed and shown schematically in figure 1.
Certain alloying elements impart characteristic and recognizable variations to the sparks given by a low carbon steel, and these variations are used to help identify the various alloys. The manner in which these various elements affect the spark is summarized in the following:
Carbon.—The presence of carbon causes characteristic bursts in the spark. The higher the carbon content the more plentiful and complicated the bursts. It should be noted, however, that these bursts can be suppressed by the presence of appreciable amounts of certain alloying elements such as silicon and chromium.
Manganese.—In steels where the amount of other alloying elements is small, manganese tends to brighten the spark and increase the spray around the periphery of the wheel. In
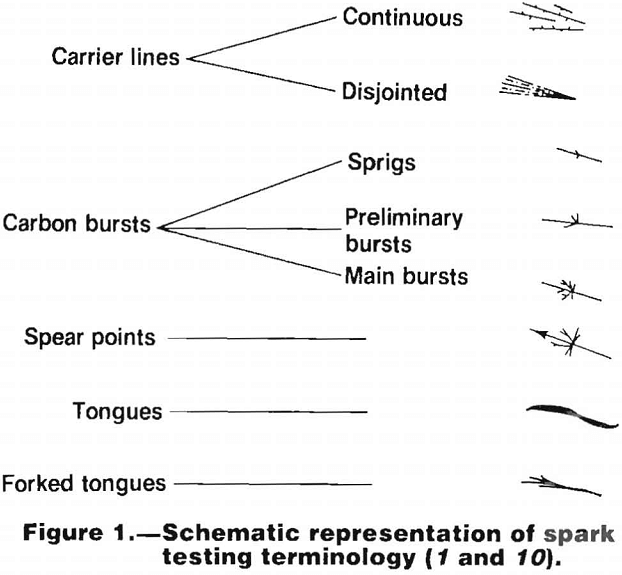
steels containing moderate or large amounts of other alloying elements, the effects of manganese are not visible in the spark stream.
Silicon.—The presence of silicon suppresses the carbon bursts. When the silicon content is greater than about 1 pct, it causes a pattern of relatively coarse fuzz (consisting of short curved lines) with a dark red discoloration close to the wheel. This effect disappears in steels containing appreciable amounts of other alloying elements.
Chromium.—Chromium suppresses the stream and the carbon bursts and imparts an orange color to the spark.
Nickel.—Nickel causes forked tongues to appear in the spark. It also suppresses the stream and the bursts slightly, although this effect is less than that caused by the presence of chromium.
Tungsten.—Tungsten tends to suppress the effects of all other alloying elements upon the spark stream. When the tungsten content is between about 1 and 15 pet, it causes single bright orange tongues at the ends of the carrier lines. The size of these tongues decreases as the tungsten content increases. In high tungsten steels, the carbon bursts are suppressed altogether. Tungsten also imparts a reddish- orange color to the carrier lines.
Vanadium.—Vanadium tends to brighten the spark stream as a whole.
Molybdenum.—In steels in which other alloying elements are not high, molybdenum causes a characteristic spear point at the end of the carrier lines. Where relatively high percentages of other elements are present, these sometimes have the effect of masking the spear points.
Descriptions and diagrammatic representations of the sparks obtained from various grades of tool steels are available (1). Of the commercially important alloys, those with iron or nickel base give characteristic sparks. Cobalt, tungsten, molybdenum, and titanium also give off sparks. All other metals are nonsparking. Brief descriptions of the sparks obtained when testing scrap have also been published and are given in tables 4 and 5.
In making the spark test, it is useful to keep on hand a set of known standards, representing the types of alloys with which the sorter is likely to come into contact, for comparison with the unknown samples.


Chemical Spot Tests
Chemical tests used for sorting or final identification of materials range from simple tests to show attack or lack of attack by specific acids, to more involved spot tests to determine the presence or absence of specific alloying elements. These spot tests are based on the formation of characteristic colors or precipitates of elements when reacted with various test reagents. These microanalytical tests are basically qualitative in nature, although semiquantitative conclusions for most of the tests that produce color reactions can be obtained by comparison with simultaneous tests on known metals and alloys. It must also be noted that in order for spot tests to be used successfully for metal identification, a knowledge of the chemical composition of the alloys likely to be encountered is required.
The lower limit of detectability of an alloying element by spot testing is generally between 0.2 and 1.0 pct, depending on the alloying element in question and the base material. For visible differences in a spot test, the quantity of the element of interest must vary in concentration by a factor of at least two, that is, 1 pct to 2 pct to 4 pct, etc. For this semiquantitative work, standard alloys are required.
Spot test procedures are designed so that maximum sensitivity and selectivity can be obtained with a minimum of chemical and physical operations. As much as possible, separation and conditioning reactions are integrated in the test procedures so that the final test becomes a unitized operation that can be applied directly for the identification of the substance in question.
In some cases the metal must be taken into solution. Reactions of given reagents with the solution are then noted. Dissolution may be accomplished by allowing reactions to take place on the metal surface or by electrographic techniques. When dissolution results from reaction on the metal surface, it is essential that a clean, degreased surface be used.
The tests are usually run using one of the following techniques:
- By bringing together one drop each of the test solution and reagent on porous or nonporous supporting surfaces such as paper, glass, or porcelain.
- By placing a drop of the test solution on a medium impregnated with appropriate reagents.
- By placing a drop of reagent solution on a small quantity of the solid material.
In the electrographic method, a sample of the material being tested is obtained by simultaneously dissolving the metal electrolytically and transferring it to a filter paper. The filter paper, wet with an appropriate solution that acts as an electrolyte, is placed on a clean degreased area of the material to be sampled. The material is then connected into an electrical circuit as the anode. The cathode is contacted to the wet area of the paper allowing a direct current to flow through the electrolyte in the paper. The anode material is dissolved electrolytically and the cations produced are transferred to the surface of the paper in contact with the material. This arrangement is shown in figure 2.
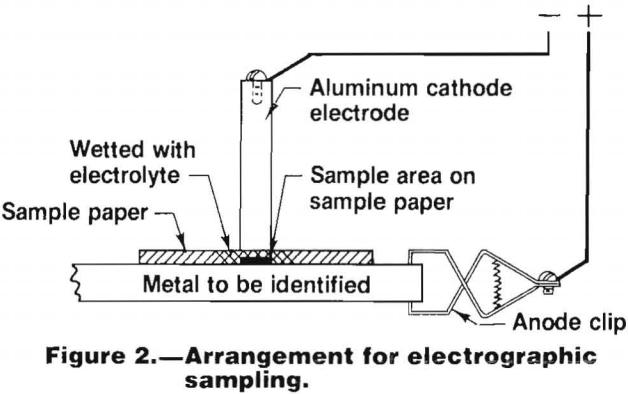
A description of an electrographic sampling device designed to sample aluminum alloys was given by Maynard and Wilson. Electrographic sampling devices are also available commercially in spot testing kits.
Detailed procedures for identification of metals and alloys by chemical spot testing have been developed by the International Nickel Company (INCO) and by the National Aeronautics and Space Administration (NASA). The tests developed by INCO have also been published by the Department of Defense.
The families of metals and alloys considered by INCO and NASA are aluminum and aluminum alloys, copper and copper alloys, magnesium and magnesium alloys, nickel and nickel alloys, stainless and heat-resisting steels, carbon and low alloy steels, and certain pure metals. In addition, the NASA system includes titanium and titanium alloys and certain tool steels.
The equipment and manipulations required for spot testing are usually simple and the techniques can be learned without difficulty. As in any chemical procedure, the user must be aware of contamination problems and safety hazards. Most procedures can be conducted by a person with no chemistry background or experience, however, some of the tests are complex and require experience to interpret properly.
In order to perform the spot tests previously referred to, the necessary reagents must be purchased or prepared by the analyst. The need for solution preparation can be eliminated by the purchase of spot testing kits in which the test solutions are already provided in plastic dropping bottles. These kits are commercially available from a number of manufacturers. Replacement solutions are also readily available. Each bottle contains enough reagent for between 250 and 1,000 tests depending on the manufacturer.
These spot testing kits vary in their range of application from tests designed for specific purposes, such as testing for molybdenum in order to separate 316 stainless steel from the other 18-8 grades, to general purpose kits that are claimed to be able to identify the majority of the most commonly used alloys.
The kits involve either electrographic sampling or spotting directly onto the metal surface, depending on the manufacturer and the application. For general purpose identification, electrographic sampling is usually used, and the kit comes complete with an electrographic sampling system. Comprehensive, easy-to-follow instructions are included with the general purpose kits. Some of the kits include a set of alloy standards, while others have alloy standards available as options.
The spot testing reagents are identified by code numbers or code names and not by chemical names so that the sorter usually does not know the reactions involved or the nature of the chemicals being used. While this is a disadvantage in the case of an accident, it helps to make the tests simpler which is an advantage for nontechnical personnel who can follow the instructions without overly worrying about understanding what is happening.
Some of the suppliers of spot testing kits and chemicals used in spot testing are listed in appendix A.
While separations can be made solely on the basis of spot testing results, a preliminary separation on the basis of weight, color, magnetic properties, etc., as discussed earlier in this report will help reduce the number of steps required by reducing the number of possibilities. Many tests involve verifying a particular metal or alloy, or separating a given material from a mixture. This enables the analyst to establish a definite starting point in performing tests, and allows the use of only a segment of the systematic procedure. In these cases, a conclusion as to the identity of the alloy can be derived quickly. Where there is no basis for selecting a starting point, the tests can take 30 or 40 min to complete.
Combining Simple Sorting Methods
The identification methods that have been discussed up to this point may be combined to form a metal identification system. The ability to combine these techniques for the purpose of metal identification requires experience, both in the application of the testing procedures used and in knowing the manner in which the various alloys behave when subjected to these procedures. In order to help the sorter, a number of charts listing many of the alloys and metals commonly encountered and indicating the behavior of these alloys under test are available.
The National Association of Recycling Industries (NARI) has included a metals identification chart in its “Recycled Metals Identification and Testing Handbook”. This chart gives the color and magnetic properties of a number of materials and also lists their response to nitric acid and ammonia. A chart of chemical spot tests for certain metals and alloys is also included. It must be noted that not all the alloys listed in the identification chart can be separated from each other. It is possible, however, to separate into groups on the basis of the tests given in the chart.
The Department of Defense, in its Defense Scrap Yard Handbook, has produced a reference table for identification of metals which is similar to the chart produced by NARI. This table also lists the response of a number of alloys to nitric acid and ammonia as well as giving color and magnetic response. In addition, the table gives nominal alloy compositions and lists typical uses of each alloy. The Defense Scrap Yard Handbook also contains a table giving color, composition, magnetic properties, sparking properties, and chemical spot tests for a number of metals and alloys. Information of this type has also been published by Obrzut.
The Institute of Scrap Iron and Steel has produced a metals identification chart for identification of common copper-base alloys and monels. This chart combines magnetic properties, color of drillings, sparking properties, and chemical tests to enable identification. Nominal compositions and typical uses of the various alloys are also listed.
Optical Emission Devices
Under suitable excitation conditions, many metallic elements emit light of characteristic wavelengths. By observing these emissions, the material may be qualitatively or quantitatively analyzed. The steps in emission spectrochemical analysis are (1) vaporization of the sample, (2) excitation of the vapor to luminescence, (3) resolution of the resultant radiation into a spectrum, and (4) observation and analysis of the spectrum.
The usual procedure for vaporization of solid samples requires the passage of an electrical discharge between two portions of the sample, or between the sample and an electrode that does not contain the elements being determined. Metal specimens having a flat surface are usually subjected to a spark discharge, with a graphite rod or piece of the sample material as a counterelectrode. Metal powders or chips may be mixed with graphite, briqueted, and partially volatilized by spark discharge. The same electrical discharge is used to produce the sample vapor and to excite it to luminescence. Radiation results from decay of the excited states produced.
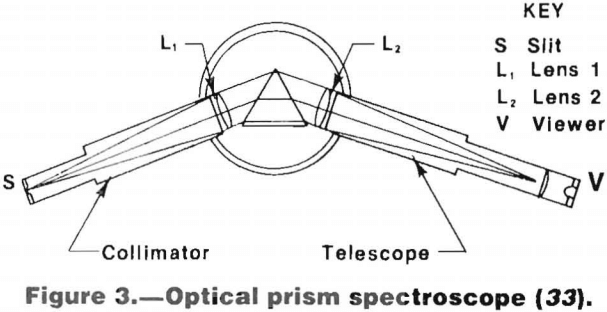
To permit analysis, the component wavelengths of this radiation are separated and arranged in order of wavelength by either a prism or grating. This is done with a spectroscope, spectrograph, or spectrometer, using visual, photographic, or photoelectric detection, respectively. The use of the spectroscope for the rapid qualitative analysis of complex materials is covered in previous Bureau of Mines reports.
A schematic diagram of a prism spectroscope is shown in figure 3.
Spectroscopes can be used for qualitative or at best semiquantitative analysis. Qualitative analysis is done by systematically trying to find sensitive lines of analysis of the supposed components of the alloy. When the lines of the spectrum have been identified, the chemical makeup of the material can be deduced. Semiquantitative analysis can be obtained by comparing the intensities of the lines in the spectrum of the sample to the intensities of samples of known composition, since the intensity of an observed spectral line increases with increasing content of the element. Semiquantitative analysis of steel can also be performed using a method known as the homologous line pair method. This empirical method is based on the fact that for steels containing a particular alloy content, the brightness of a particular spectral line of the alloying element is similar to that of a neighboring iron line. Both these lines form what is known as a homologous pair and, by consultation with tables, an estimate of the alloy content of the steel can be made.
For semiquantitative analyses or for alloy confirmation, comparative spectroscopes, in which the spectrum of the sample is compared with that of a standard of known composition, may be used. A number of such instruments are available commercially. These instruments have two sample tables: an analysis sample table that bears the unknown; and a standard table that bears a comparison sample, which is either a piece containing the nominal contents of the alloy in question or a piece of the pure base metal of the alloy. The sample and the standard are arced simultaneously with the light from both being reflected into the spectroscope. The spectra of both appear juxtaposed in the eyepiece allowing easy comparison of the two for both position and intensity of the spectral lines.
The limits of detection of a spectroscope depend on the instrument, the element being sought, and the base material or matrix. In steels, the lower limit of detection for the elements chromium, manganese, molybdenum, titanium, vanadium, and copper is about 0.2 pct. Cobalt, nickel, and tungsten can only be detected above about 1 pct because of
interference from adjacent iron lines. Silicon, aluminum, and niobium cannot be detected visually as their main verification lines are not in the visible range. Limits of detection in nonferrous materials also vary over a wide range.
Commercially available spectroscopes vary greatly in size and mobility, ranging from hand-held portable units to mobile units on wheels and up to larger instruments that must be installed in either a workshop or a laboratory.
Concurrent with changes in the mobility of the instrument, the sampling needs change. For a portable instrument, no sampling of individual pieces is required as the instrument can be taken to the material, provided there is a suitable power outlet in the vicinity. Little or no surface preparation is required, although thick oxide films should be removed. The mobile unit can accommodate samples up to the size of an ingot, although the sample must be placed on the sample stand and this may be more easily accomplished if a sample is cut from the material. For the installed units, a sample must be cut from the material. Since the sample usually sits on a table and is sparked from below, it should have a flat surface. For these instruments, therefore, sampling is an important factor. Since only a small area is tested, the homogeneity of the material also affects the reliability of the analysis.
Cleanliness of the electrode is also important. When a material is sparked, the vapor generated coats the electrode. This takes a finite time to be burned off during the next test. Care must be taken, therefore, to ensure that the spectrum observed belongs to the material being tested and is not due to residual effects on the electrode.
When the arc is struck, a minute amount of metallic oxide fume is given off from the sample surface. However, the levels of fume will normally be well below safety limits so that no health risks exist to the operator, although it is possible that repeated sparking of material containing a high level of toxic elements such as lead could produce a problem. In addition, the arc formed by the electrode should not be viewed directly with the naked eye for a prolonged period.
Extension of the wavelength range into the ultraviolet region is an advantage. The lines of highest intensity are usually found in this region. In addition, a number of elements have spectral lines that appear only in this region. The use of wavelengths down to 200 nm (nanometer) permits detection of all metals except small amounts of the alkali metals. Because this region is beyond the range of the human eye, photographic or photoelectric means must be used to detect the spectral lines.
A relatively new advance in the application of optical emission devices to metals identification has been the development of small, mobile spectrometers that can be moved around yards for onsite testing. The penalty for portability is that the sensitivity and resolution possible with large, expensive spectrometers is decreased. The capabilities of the mobile spectrometers are, however, quite adequate for alloy sorting and identification.
The basic operation of the available portable instruments is similar, although the detection capabilities differ. Sparking is achieved using a pistol (Aerosol Generator Capillary Arc Pistol) that eliminates the need to cut samples from the test piece. A direct current arc is ignited between a counterelectrode, located in the front part of the pistol, and the metallic sample material. To do this, the end of the pistol barrel is placed against the metal, and a single trigger pull initiates a low voltage between the electrode and the metal surface. The emitted light travels along a fiber optics cable up to 10 m long to the entrance slit of the spectrometer optics. The light is separated into discrete wavelengths by a grating. Selected spectral lines impinge on preadjusted slits with photomultipliers mounted behind them. In this way, a number of elements can be detected simultaneously.
The portable instruments are strictly comparators. A reference standard is sparked and the intensities internally stored. When the test material is sparked, the intensities of the emission are compared to those of the standard.
Accordance between test and reference materials within preset tolerances is displayed by a green light, differences by a red light. Testing takes 10 sec or less and no technical experience is necessary.
These instruments have been designed primarily to verify the composition of batches of steel mill products. They can be used for final metal identification, particularly if the number of possible alloys is small. They may also be adapted for use with nonferrous alloys.
A number of optical emission devices are commercially available that vary greatly in cost and applicability and in the experience and skill required to operate them. A list of suppliers is included in appendix A. The main features of these instruments are summarized in appendix B.
X-Ray Emission Devices
X-rays are electromagnetic radiation of the same nature as light, but with much shorter wavelengths, occupying the region between gamma rays and ultraviolet radiation. For analytical purposes, the wavelength range of interest is from 0.036 to 2.5 nm. Only the shorter wavelength portion of this region is transmitted in air; for wavelengths above about 0.3 nm, a vacuum path must be used.
X-rays are produced when high-speed electrons collide with a target material. This is normally accomplished in an X-ray tube that contains the filament and target in an evacuated enclosure. Electrons from the filament are accelerated to the target by a high voltage applied across the field. This voltage is of the order of 30 to 50 kV. These accelerated electrons collide with the metal target and produce X-rays at the point of impact. X-rays can also be produced using other charged particles, gamma rays, and other X-rays.
If the X-rays generated are sufficiently energetic and are used to strike a material, an inner shell electron can be ejected from an atom, leaving the atom in a state of high potential energy. This is the excitation process. The vacancy is quickly filled by an electron from an outer shell, accompanied by the emission of an X-ray photon or an Auger electron. The energy of the emitted X-ray is characteristic of the specific electron transition.
Some of the X-rays incident on the sample are simply scattered by the atoms of the sample, with or without a loss of energy. All of the X-rays emerging from the sample form the emission spectrum.
When X-rays are used to excite the atoms of the elements in the sample to be analyzed so that their characteristic radiation spectra are emitted, the process is known as X-ray fluorescence or secondary emission. This is the process that was previously described. Alternatively, the X-rays may result from the impact of electrons on the surface of the sample. This is known as primary X-ray emission. It is also possible to use gamma rays for the stimulation of characteristic X-rays from the sample. In this case, a radioisotope, emitting gamma rays, is substituted for the X-ray tube.
The irradiation of a sample containing a number of elements by an exciting X-ray or gamma ray source results in the emission of many characteristic X-ray wavelengths. In order to analyze the sample, these have to be separated. Since all the characteristic X-ray wavelengths from the elements are known, identification is possible. Moreover, since the intensity of the radiation is a function of concentration, it is possible, after suitable calibration, to quantitatively determine the composition of the sample material. Extraction of the desired information from the emission spectrum involves X-ray spectral analysis, data processing, and interpretation.
There are two basic methods of X-ray spectral analysis: wavelength dispersive and energy dispersive analysis. Wavelength dispersive analysis refers to the separation of the X-ray spectrum into wavelengths by using a crystal. This is accomplished by allowing the X-rays from the sample to be diffracted by a crystal of known interatomic spacing and crystallographic orientation.
Energy dispersive X-ray spectroscopy is a spectral analysis technique whereby the X-rays generated from the sample are separated and measured by means other than crystal dispersion.
There are three basic types of detectors, with different resolutions, used in energy dispersive X-ray spectroscopy. The resolution of the system is a measure of the degree to which X-rays slightly separated in energy can be distinguished; that is, a measure of the spread of the pulse amplitude distribution. The detectors with the best resolution are the solid-state detectors. Either lithium-drifted silicon, Si(Li), or intrinsic germanium are used. These have resolutions of the order of 150 eV at 5,900 eV. They require special maintenance, including operation at cryogenic temperatures. Solid-state detectors have a broad energy detection range.
Next best in resolution is the gas-filled proportional counter, which has a resolution of about 1,000 eV at 5,900 eV. The gas proportional counter is often similar to the Geiger counter in construction and filling, except that the proportional counter uses a lower voltage and the size of the output pulse is proportional to the number of initial ionizations caused by a particular X-ray quantum.
The third type of detector is the scintillation counter that has a resolution of approximately 3,000 eV at 5,900 eV. It consists of material such as thallium-activated sodium iodide that converts a fraction of the X-ray energy into visible light. The light is detected by a photomultiplier and transformed into electrical pulses. The output pulse distributions from scintillation counters are relatively broader (two to three times as broad) than those from proportional counters. Consequently, there is more overlap of neighboring photons and poorer resolution of elements with scintillation counters. Pass-band filters (thin foils), which absorb some wavelengths more strongly than others, are often used to improve resolution when using a scintillation detector.
The principal advantages of energy dispersive X-ray systems are simpler instrumentation and high intensities. The major disadvantage of the energy dispersive method of analysis is the relatively low energy resolution. This results in difficulty in resolving neighboring elements. The resolution of elements with two or three atomic numbers between them is only partial so that difficulty may be experienced in determining minor concentrations of elements.
As with optical emission devices, large X-ray fluorescent spectrometers are in widespread use in the metals industry for monitoring the composition of alloys in various stages of production. Smaller, less expensive portable or semiportable machines are also available, although their detection capabilities are reduced. These can be used for metal sorting and alloy identification in storage yards or in the field. Those X-ray emission instruments that have been designed specifically for metal sorting and alloy identification are comprised of two basic components: a probe and an analysis unit.
The probe, which is often hand held, contains the primary radiation source and the detector. Either an X-ray tube or radioisotope may be used as the primary source, although most modern instruments use radioisotopes. The radioisotopes are often interchangeable, depending on the application, because the range of elements detectable depends on the radioisotope being used. Some of the commonly used radioisotopes include iron-55, cadmium-109, americium-241, cobalt-57, and curium-244. Field X-ray techniques cannot generally be used to determine elements with atomic numbers lower than 19 (potassium), although in certain cases aluminum and silicon may also be detected. The detector also varies from instrument to instrument. Instruments using crystal dispersion techniques are also available.
The probe is connected to the analyzer by a signal cable which can be up to 50 ft long. The pulse from the detector is passed into analog electronics to select for digital processing those pulses that represent the specific elements of interest to the user. This information is converted into the form needed by the operator.
By the use of microprocessing units in which a number of alloy compositions are stored, alloy identification can be made by the instrument if the material being tested corresponds to one of the stored alloys. The alloy to which the sample corresponds is indicated on a display.
In order for accurate analysis or identification to be made by X-ray emission analysis, the instrument must be calibrated. Since the aim of X-ray analysis is to determine the composition of the sample, it is usually convenient to establish a calibration in which the intensity of a characteristic X-ray line is a function of the concentration of that element in a multielement matrix. This calibration is sensitive to effects of the other elements present and must be corrected for these interelement effects. Because of the sensitivity of the method to these matrix effects, only those materials with compositions in the range covered by the calibration can be accurately analyzed. For materials other than these, the instrument must be recalibrated. The calibration may be carried out by the supplier in accordance with the user’s requirements or may be done by the user.
Certain hazards are associated with X-ray devices. While all the instruments commercially available are safe when operated correctly, the user must observe all necessary safety precautions. In certain cases a Nuclear Regulatory Commission license is required. Devices using radioisotope sources generally have a shutter over the source which opens when the probe is in contact with the sample. Malfunction of the shutter could result in a safety problem, for the radioisotope sources cannot be turned off, as compared to the X-ray tube source.
The main features of those devices suitable for metal identification are summarized in appendix C. A list of suppliers is included in appendix A.
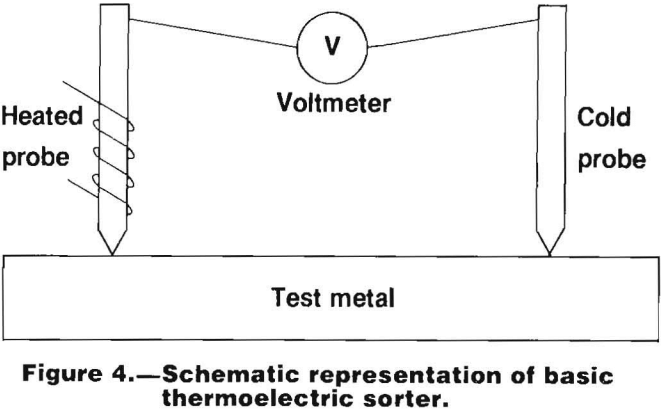
Thermoelectric Instruments
A heated junction between two dissimilar metals will produce a potential difference. This is known as the Seebeck effect, and is widely used for temperature measurement and control. It is the basis of thermoelectric instruments that are finding an increasing application in the fields of metal identification and sorting.
The magnitude of the potential difference produced depends on the temperature difference between the two junctions and on the nature of the metals involved. This means that if the temperature difference is held constant, and if one of the metals forming the junction is also held constant, the potential difference obtained will depend on the nature of the other metal in the junction.
Some alloys will produce potential differences that vary greatly from each other, while other alloys will have similar readings. This can often be altered by changing the other junction material.
The potential difference obtained depends, in addition to temperature and chemical composition, on the physical nature of the test material. This presents a problem because it means that the potential is sensitive to such things as structure, hardness, and surface conditions. Thermoelectric measurements are, therefore, sensitive to heat treatment, cold working, etc.
A thermoelectric sorting device measures the potential difference between an unknown surface and a heated, known surface. Such an instrument must incorporate the following features shown schematically in figure 4:
- Two points of contact on the sample.
- A heat source so that one point of contact will be hotter than the other point of contact.
- A sensitive direct current voltmeter, either analog or digital, and calibrated either directly in microvolts or in relative units.
There are three ways in which such an instrument may be used for metal identification. The first of these is an absolute measurement of the voltage, in microvolts, that is generated, A table can be constructed listing the microvoltages for each alloy that is likely to be encountered. Both chemical composition and structure must be considered. In this mode of operation the microvoltage is a function of the temperature difference between the junctions, and any inaccuracies in
controlling the temperature will produce variations in the microvoltage readings.
In the second method, the voltage is used to generate a relative number over the range of alloys likely to be encountered. As with the absolute measurement, a table can be constructed listing each alloy and temper and its metal sorter number.
The third method is used for confirmation. This requires a set of standard samples to calibrate the instrument and a standard sample of the alloy being sought. The instrument is calibrated for proper operation by checking a standard with low thermoelectric voltage and a standard with high thermoelectric voltage for proper readings. A verified piece of the material sought is then tested and the instrument reading adjusted to either zero or some other convenient voltage. Any sample that shows a reading other than the set value is not of the material being sought.
The major advantages claimed for thermoelectric identification are the speed with which a test can be accomplished and the inherent nondestructive nature of the process. Because of the geometry of the probes, the junction of the two metals is usually small and, hence, the test temperature is reached very quickly, provided the instrument has warmed up and the probe tip has attained operating temperature.
After the instrument and probe have come to equilibrium, a test can be accomplished in several seconds. The method is independent of geometry and mass, provided that the material is not excessively thin or small, thus no sampling is required.
The traditional problem with the thermoelectric process for alloy separation is one of controlling the junction temperature closely enough to establish a stable, reproducible reading. It is electronically possible to resolve a few microvolts at the junction at reasonably low temperatures. A change in temperature as small as 0.5° C, however, will produce as much at 10 to 20 µV change in output. The junction temperature may fluctuate several degrees, even with sophisticated control, and this must be compensated for electrically. This is complicated both by the difficulty in measuring the actual junction temperature, and more significantly, each material tested requires a different level of compensation owing to differences in thermal conductivity.
The first commercial instruments using thermoelectric effects for alloy sorting were available in the early 1960’s. Some instruments read out in microvolts, others in relative units. Some instruments read out in microvolts, others in relative units. Some used copper electrodes for all testing while others provided special alloys for various testing problems. The method of contact varied from two similar probes, to a heated probe and a large ground plate, or a heated probe and a large alligator clip. These variations in design still exist in the current commercial instruments.
The manner in which the problem of temperature control is treated also varied. There are two main strategies in use, although the means by which they are implemented vary. The first of these controls the temperature of the probe tip and, hence, the hot junction at a given value. The second method is to maintain the difference in temperature between the hot and cold junctions at a constant value. This latter method eliminates the need for recalibration to account for changes in conditions.
An interesting approach to this problem is that of Rowsey. In this instrument, the need for maintaining a precise temperature at the hot junction or a precise temperature difference between the probes is avoided by relying not simply on the absolute value of the electromotive force generated between the probes and the unknown sample, but, on the relationship between that value and an electrical signal that is indicative of the temperature difference between the probes. This latter signal can be derived from thermocouples attached to the probe tips. Any change in temperature difference between the probes will result in variations in both signals. The ratio of the two will be substantially constant despite minor variations in the temperature difference between the probes and is determined by a potentiometric bridge circuit.
In order to obtain reliable results using thermoelectric devices, it is necessary that the surface of the material being tested should be clean and free from scale. It is essential that the base metal and not a coating be tested, because the reading obtained depends on the nature of the material at the point of contact with the probe. The surface can be cleaned by rubbing with emery paper and wiping off the residue with a clean cloth. The probe can become contaminated with material from the surfaces with which it comes into contact, and should be cleaned periodically. This is especially important when testing easily oxidized metals such as aluminum.
The probe tip temperatures of the commercially available thermoelectric instruments range from approximately 70° to 350° C and thus represent a safety hazard. The instruments can be operated by personnel with no technical training, after instruction in their safe use.
The operating characteristics of a number of commercial instruments are given in appendix D. A list of suppliers appears in appendix A.
Eddy Current Testing
An eddy current is a circulating electric current induced within the body of a conductor when that conductor either moves through a nonuniform magnetic field or is in a region where there is a change in the magnetic flux. Typical currents of this sort resemble in form the eddies in flowing streams of turbulent water, hence, the name eddy currents. Although they can be induced in any electrical conductor, the effect is most pronounced in solid metal conductors. A suitable magnetic field can be generated by a coil carrying an alternating current. This magnetic field will interact with a test object brought near to the coil causing eddy currents in the test object. These eddy currents in turn create their own electromagnetic field, which may be sensed either through its effects on the primary excitation coil, or by means of an independent sensor.
In nonmagnetic materials, the secondary electromagnetic field depends simply on the eddy currents. With ferromagnetic materials, additional magnetic effects occur that usually are much larger than the direct eddy current fields. These magnetic effects result from the magnetic permeability of the test material.
The main factors in determining the magnitude and direction of eddy currents induced by harmonically varying magnetic fields are as follows:
- The geometrical shape of the applied field.
- The amplitude of the applied field.
- The frequency of the applied field.
- The size and shape of the test object.
- The location and orientation of the test object with respect to the applied field.
- The electrical conductivity of the test object.
- The magnetic permeability of the test object.
A very small change in any one of these can have a marked effect on the eddy current produced. For practical testing purposes, it is usually necessary that all of these parameters be rigorously controlled.
From the point of view of metal identification, the factor of most importance is the effect of electrical conductivity. Since the electrical conductivity of an alloy is sensitive to changes in chemical composition, eddy current techniques can be used to identify metals and alloys. Electrical conductivity is, however, sensitive to changes in the physical nature of the material. This is a complicating factor when this technique is applied to scrap metal identification where a given alloy may be present in different physical forms.
Eddy currently test equipment ranges from simple portable units to complex automatic or console-type apparatus. Regardless of the complexity, however, each system must have at least the following elements:
- A source of magnetic field capable of inducing eddy currents in conductive materials.
- A sensor or transducer, sufficiently sensitive to detect the very small changes in the magnetic field caused by eddy currents.
- A means of interpreting the measured changes in the magnetic field, whether it be by monitoring a meter whose reading is proportional to the magnetic field change or an electronic black box that displays readings proportional to phase magnitude or modulation of the magnetic field.
In simple eddy current instruments, the voltage across the coil may be used as a measure of the eddy current effect and may be manipulated to produce a meter reading’Indicative of the desired property or feature under investigation. There are many variables active in eddy current testing that affect the results. In application of this technique to alloy sorting and identification, changes in electrical conductivity are usually used as a basis for identification. However, the impedance values observed can be influenced by heat treatment, grain size and orientation, grain boundary precipitation and geometrical factors, among others. This means that identification of materials may be ambiguous.
Eddy current testing is rapid, nondestructive, and can be substantially automated. In addition, mechanical contact with the test article is not normally required, although, in most cases, it is necessary to have a flat surface. On the other hand, the number of variables that may influence the result often make the interpretation of the test results ambiguous.
The method is restricted substantially to surface analysis owing to skin effects, so the presence of coatings, scale, etc., presents a problem. When the test articles are ferromagnetic, the permeability of the test specimen changes as it is brought near to the coil which may obscure the results.
The eddy current method is best suited to testing large numbers of mass-produced articles. The response of a standard article may be stored in the memory unit of the instrument. The response of the test articles is then compared to the stored standard and either accepted or rejected. In this way, the articles may be tested for correct composition and also for flaws on or near the surface. Eddy current testing, at its present state of development, has very limited applicability for identification of mixed scrap materials owing to the complicating effects of shape and structure.
A list of manufacturers of eddy current instruments is given in appendix A.
Quantitative Chemical Analysis
Positive identification of alloys can be made by performing a quantitative chemical analysis and then referring to specification tables giving chemical composition for various groups of alloys. Often it is necessary to determine only a small number of elements in order to identify the alloy, but for more complex alloys a more detailed analysis will be required.
Quantitative chemical analysis may be made using classical wet analytical techniques, instrumental techniques, or a combination of wet and instrumental methods.
Wet chemical analyses, if performed correctly on properly taken samples, are very accurate. They may be, however, less sensitive and more time consuming than instrumental analyses. When doing wet chemical analysis, it is important that standard methods of analysis be used. Standard methods for chemical analysis of metals have been published by the American Society for Testing and Materials (ASTM). These give details of the reagents and procedures to be used for determining elements in alloy groups.
A number of instrumental techniques may be used for quantitative analysis. These include optical emission analysis and X-ray analysis, the principles of which have been discussed elsewhere in this report. In the context of quantitative analysis, the instruments used are, in general, larger, more complex, and more expensive than those previously described.
A technique now in widespread use in analytical laboratories is atomic absorption analysis. If light emitted by an element inside a special hollow cathode lamp is passed through a gaseous cloud containing that element in the atomic state, then the atoms of that element, and only that element, will absorb the light, in practice, the gaseous cloud is formed by aspirating a solution of the sample to be analyzed into a flame of sufficiently high temperature to reduce the element to its atomic state. The amount of light emitted from the lamp that is absorbed by the aspirated sample is proportional to the amount of that element present in the solution. Comparison with the absorbance of known standards enables the amount of the element in the sample to be determined. This analysis can be greatly accelerated by the use of specially designed graph paper or by means of computer programs. Advances in instrumentation has made even more rapid analysis possible. Prior to analysis, the sample must be taken into a solution of known volume with suitable solvents. For metals, this step is normally straightforward. Atomic absorption analysis, when properly applied, has few disadvantages and is fairly rapid, accurate, and the equipment is comparatively inexpensive.
A more recent development is the inductively coupled plasma optical emission quantometer. These instruments analyze samples by producing an aerosol of the sample and exciting it in a very stable argon plasma at temperatures from 2,000 to 10,000 K. Diffracted spectral light from the excitation is measured as in conventional optical emission devices. This method has the advantages of better detection limits and the capability of measuring a wider range of emission intensities (hence a wider range of concentrations) since linear calibration curves are obtained.
In all quantitative analysts, it is essential that the sample being analyzed be truly representative. Sampling procedures , therefore, are very important. Standard sampling methods for various materials have been published by ASTM and serve as a guide when sampling scrap material. The methods used range from clippings or drillings taken from selected pieces to melting a proportion of the material and taking drillings from the resultant ingot. In order to ensure uniformity of the sample, the sampling method must become more complex as the material being sampled becomes less uniform in nature.
Other Potential Methods
There exist a number of well-established techniques that are used for applications other than metals identification, but which could possibly have applications for identifying and separating certain metals and alloys. While the manufacturers of these instruments do not claim that they can be used for metals identification, the potential does exist, and this report would thus be incomplete without referring to them.
Colorimetry
Colorimeters are used extensively in the textile, paint, food, printing, and similar industries for determining color differences with greater precision than the human eye. Recent work on the measurement of alloy color has been reported. This work was directed towards areas where alloy color is a desirable property and was designed to provide a method of quantifying alloy color and color stability in tarnishing environments.
Perception of color depends on three elements: the light source, the object, and the observer. To quantitatively characterize the color of an alloy, it is necessary to standardize both the light source and the observer. For color measurements, the light source is characterized by the spectral energy distribution over the optical wavelengths. Blackbody light sources are used since their spectral energy distributions can be specified by temperature. The standard observer is normally a scanning spectrophotometer.
By itself, spectral energy distribution information in a graphical form of reflectance as a function of wavelength is difficult to interpret. However, color can be specified in terms of numerical indices. The most widely used color system is the CIE system (developed by the Commission Internationale de I’ Eclairage). The CIELAB uniform color scale, on which the CIE system is based, measures the three- dimensional color space components lightness, red-green, and yellow-blue.
It is a fully quantitative system based on opponent colors. The units on all three scales are representative of approximately equal increments of color difference so it is a uniform color space. It is a rectangular coordinate system that has a meaningful relation to human visual perception of
color differences. The lightness, red-green, and yellow-blue components are all available as direct readouts from color instruments.
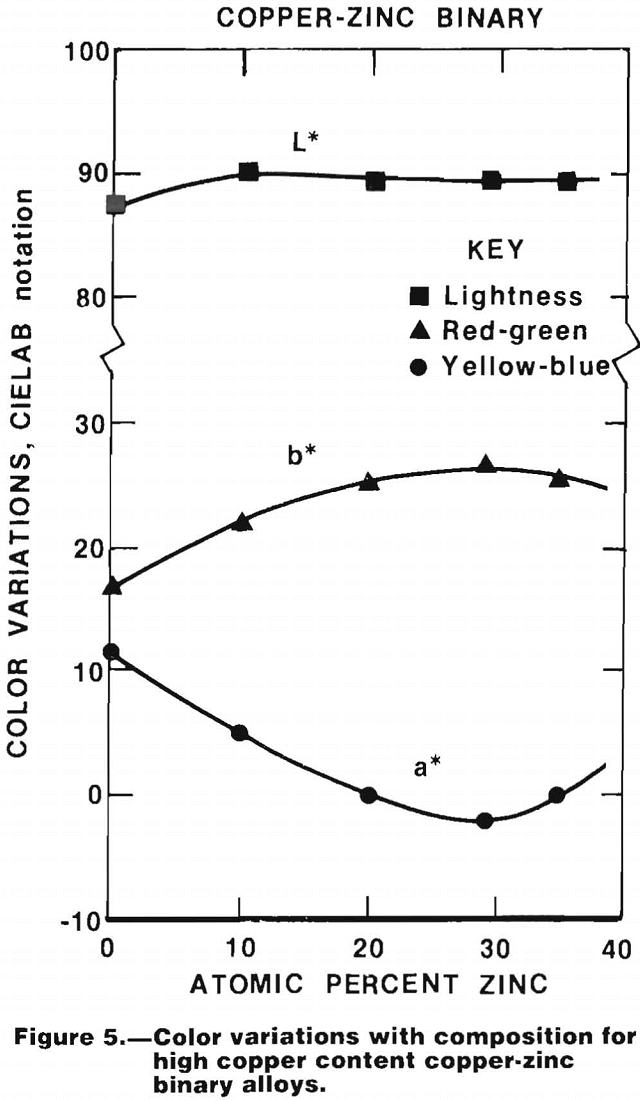
The results of color measurements on a series of copper-zinc alloys cast into 10-by 10-by 1-mm paddles that were ground and polished before testing are shown in figure 5. The potential of colorimetry for identification of certain copper-base alloys can be readily seen. The potential of this method has also been demonstrated by the Bureau of Mines.
Color measurements are affected markedly by the method used to prepare the sample surface. Some of these effects are peculiar to certain regions of the color spectrum; whereas others are not limited to any particular color region. An example of the effect of surface preparation is the amount of lead streak produced in alloys containing that element, which in turn affects the reflectivity of the metal in the blue regions.
Besides the need for careful surface preparation, commercially available colorimeters have two major deficiencies when applied to scrap sorting. The sample areas required are relatively large because the optical efficiencies of the instruments are not high, and the instruments are designed for use under laboratory conditions. Both of these may possibly be overcome by the use of fiber optics systems for the incident and reflected light paths.
Ultrasonic Inspection
Ultrasonic inspection makes use of mechanical waves above the audible range. Because the ultrasonic waves are based on mechanical phenomena, they are particularly useful for determining the integrity and structure of materials. In addition, ultrasonic energy can be readily introduced into materials, and the resulting wave motion is easily transmitted.
An ultrasonic beam impinging on an interface between two media, for example, the test object and its surroundings, is partly reflected and partly transmitted in accordance with well-known physical laws. The characteristic that determines the amount of reflection is the acoustic impedance which is the product of the density of the medium and the velocity of the sound waves within it.
Metals and alloys of different compositions have different impedances. This then gives rise to a possible identification method based on the propagation of ultrasonic waves in the material. Such an identification method would, however, be greatly affected by the internal structure of the material being tested, for example, a flaw can be detected as its impedance is different from that of the surrounding materials.
Acoustic Emission
Acoustic emission is the energy that is released when a material is stressed either gradually or suddenly. The energy is released in the form of a sound wave that travels through the material from the point of origin to the limits of the structure. The energy of each pulse is characterized by a frequency of about 40 kHz, which is well above the frequency of audible sound or vibration.
The acoustic emission coming from a material under stress can be sampled by means of a probe pressed against the material. The probe is a transducer, which changes the shock wave energy into electrical waves. This electrical signal is transmitted to the electrical measuring system which rejects the vibration and sound portions of the probe signal and amplifies the acoustic emission for measurement and display on a decibel meter. The size of the reading for a given material is a measure of the strain being produced in the metal.
Because different materials behave differently under stress, and because sound waves propagate at different rates in different materials, it is possible that acoustic emission could be used in metal identification. As with ultrasonic methods, acoustic emission is very sensitive to the physical and structural condition of the material. The full potential of acoustic emission has not yet been realized as it is a relatively new field.
Magnetic Permeability
The magnetic permeability, µ, is a characteristic parameter of a material. It is usually convenient to define another quantity, known as the relative permeability, as the ratio of the permeability of the material to the permeability of empty space. Materials may be classified in terms of their relative permeabilities. Diamagnetic materials have relative permeabilities a little less than unity; paramagnetic materials have relative permeabilities a little greater than unity. Ferromagnetic materials are those that have relative permeabilities considerably greater than unity.
Magnetic permeability is a function of composition, and, thus has some potential as a means of identifying metals and alloys. Differences in magnetic permeability are, in fact, used in preliminary sorting with a hand magnet, although in this case no attempt is made to assign quantitative values to the permeability. Unfortunately, magnetic permeability of ferromagnetic materials is greatly influenced by the past magnetic history of the material. The permeability of paramagnetic and diamagnetic materials is greatly influenced by the presence of ferromagnetic impurities. The greatest potential for the use of magnetic permeability appears to be in the identification of slightly ferromagnetic materials.
Magnetic Susceptibility
Magnetic susceptibility describes the magnetic response of a substance to an applied magnetic field. Since magnetic susceptibility varies with composition among other factors, it is possible that use could be made of differences in magnetic susceptibility for identification. Commercial instruments are available for measuring the volume magnetic susceptibility of mineral drill cores, hand samples, or outcrops. It is possible that they could be adapted for use with metals.
Infrared Emission
The infrared portion of the spectrum covers wavelengths from about 750 to 10 6 nm. These are the wavelengths between those of visible light and the microwaves used in the highest frequency radar systems. For convenience, this band is said to consist of the near infrared (750 to 1,200 nm), the intermediate infrared (1,200 to 7,000 nm) and far infrared (7,000 to 10 6 nm) regions. Infrared radiation is naturally emitted by all objects because of the thermal agitation of their molecules. This motion increases as the temperature of the object increases and decreases as the temperature decreases until it stops at absolute zero. Since all molecules are made up of electrical charges, the oscillations of these molecules cause the radiation of electromagnetic energy. The intensity, frequency, and wavelength of this electromagnetic energy are controlled by the temperature and size of the source and by the emissivity of the material. The emissivity is the ratio between the radiation emitted from a body and the radiation emitted from an equivalent blackbody. The value of the emissivity varies with the material and the surface finish of the body.
Since the composition of the material affects its emissivity, and hence the amount of radiation energy emitted from a body at constant temperature, some potential exists for the application of emissivity in metals identification. However, surface finish and shape play a greater role so careful surface preparation would be required.
Three fundamental types of infrared instruments are commercially available. Infrared thermometers make non- contact temperature measurements of the object area of interest. Process control instruments measure the temperature of the object or area of interest and generate a control signal to maintain that object or area at the desired temperature. Thermographs, also known as infrared cameras, scan a large area of interest and form an image that shows the varying amounts of infrared radiation being emitted by different parts of that area. These instruments could possibly be adapted for metals identification.
Galvanic Measurement
A difference in electrical potential always exists between two dissimilar metals. If these metals are placed in contact or otherwise electrically connnected, this potential difference produces electron flow between them. The current is a function of the composition of the two materials and thus provides a method of metal identification.
A comparative method using galvanic measurements for distinguishing type 316 stainless steel from Durimet T (22 pct Ni, 19 pct Cr, 2.5 pct Mo, 1 pct Cu, balance Fe) has been described by the International Nickel Company. These two alloys cannot be distinguished by standard hydrochloric acid and sulfurous acid chemical spot tests because they react similarly. In the galvanic method, a known specimen of one alloy and the unknown specimen are immersed in a 10-pct HCL solution and are connected to the terminals of a 0 to 1 milliampmeter. No permanent deflection of the ampmeter needle identifies the known and unknown specimens as the same alloy. A permanent deflection of the needle identifies the unknown specimen as a different alloy than the known specimen. Similar methods could be used for other alloys.
Summary
A large number of methods and instruments used in identifying metals are compared in appendix E. Unfortunately, there is no one method or instrument capable of rapid and accurate identification of every combination of composition and physical condition. Complete chemical analyses, when properly performed, will give the required accuracy but require long times, a high degree of operator skill, and expensive laboratories and equipment. Manual and instrument methods with the required speed of identification often lack accuracy or versatility.
Most of the identifying instruments have been developed for use in confirmation of identity; that is, they are basically comparators. They work well in this context to confirm both the identity and correct treatment of wrought and cast stock and finished articles withing their analytical capabilities. When used for scrap identification, complicating factors such as changes in the physical character of the material and the presence of dirt, grease, platings, and corrosion products may affect the operation of the instrument. In addition, the need is more for an identifier rather than a comparator, as the range of materials likely to be encountered is greater and varies more rapidly than in a production situation. These problems are greatest for obsolete scrap, as identification of prompt scrap may be greatly facilitated by a knowledge of, and cooperation with, the source of the material.
The suitability of the methods and instruments described in this report for a given application may be gaged from experience and manufacturers’ data. Confirmation requires testing of appropriate samples. Many manufacturers offer a service in this regard and some will dedicate their instruments at the factory to fit the customers’ requirements.
