Table of Contents
Here is a comparison of two Hydroxamate Collectors used for the flotation of Oxide copper, namely CTC3 from OXFLO and AM2 (AKA Rinkalore) from AXISHOUSE. This report contains a comparison of the scoping test-work done by both suppliers to determine which one is the best Oxide Collector .
Test Summary #1
Flotation by Hydroxamate: OXFLO’ CTC3 vs XANTHATE
911Metallurgy Corp started a lab testing program with Oxflo (Pty) Ltd – Mineral Processing Reagents to evaluate improving copper oxide recoveries at an oxide mine. Samples were shipped to South Africa for scope testing. Below are early stage tests comparing Xanthate to the Oxflo’s Activator/Collector which show a clear advantage to Oxflo.
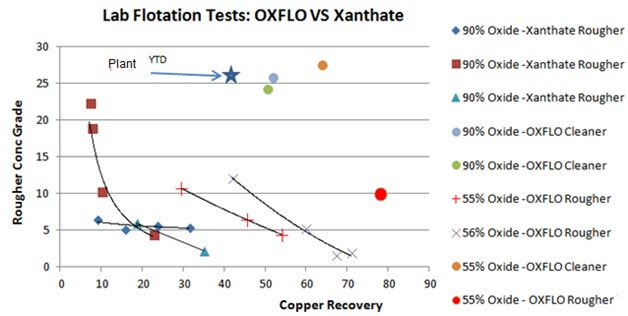
Comparative Laboratory tests between OXFLO and Classic XANTHATE on heavy oxide material always showed metallurgical improvements with early results of 20% to 40% Copper Recovery along with up to 20% Silver Recovery increases. The recovery of Gold was never negatively affected.
Why is %Mass Recovery/Pull important
Comparing Hydroxamate Collectors
Compare Hydroxamate AM2 Rinkalore CTC3 Oxide Collectors
Terminology
“Oxide” copper is a general term used to describe non-sulfide copper minerals found in oxidized zones of porphyry copper deposits. These non-sulfide copper minerals include malachite [Cu2(CO3)(OH)2], pseudomalachite [Cu5(PO4)2(OH)4], azurite [Cu3(CO3)2(OH)2], chrysocolla [(Cu, Al)2H2Si2O5(OH4)nH2O], cuprite [Cu2O], atacamite [Cu2Cl(OH)3], paratacamite [Cu2(OH)3Cl], tenorite [CuO] and native Cu. All of these minerals are referred to in this paper as “Well- defined oxide copper minerals”.
“Acid Soluble copper” (abbreviated AS Cu in this paper), “Non-Sulfide copper (NS Cu)”, “oxidized” copper (ores or minerals) are terms used in the industry to describe “Oxide Copper” minerals. All of the terms are rather vague and none of them clearly defines the various copper species present in the ore. In this paper, these terms are often used interchangeably, but preference is given to AS Cu because the chemical assays obtained for “oxide” copper were based on dilute acid digestion of the ore. Hydroxamates, Alkyl hydroxamates and AERO 6493 Promoter are used interchangeably.
Materials and Methods
Ores
Natural ore flotation was the main focus of this study [single mineral studies are adequately described in the literature]. Ore samples, from various operating mines and new mines, were used in this study. The description of the ore is given in the respective case studies. For some ores the flotation tests were conducted at the mine’s laboratory using the plant recycle water, and for the others the tests were conducted in the Stamford Laboratories of Cytec Industries Inc. using tap water.
Collectors
The sulfide collectors and frothers, and their dosages, were the same as those prescribed by the individual plant. Alkyl hydroxamate collector was the commercial sample AERO 6493 Promoter manufactured by Cytec Industries Inc.
Flotation
For laboratory flotation tests, 0.5 or 1 kg of ore was ground in a 0.24m diameter mild steel rod mill with 9.5 kg of mild steel rods at 67% solids to obtain a granulometry similar to that reported by the plant. Lime was added to the mill for pH control. The ground slurry was then transferred to a Denver cell of appropriate size, pulp level adjusted with tap water to provide -2.5cm froth depth (pulp density -35%), conditioned with appropriate dosage of sulfide collector and frother and floated to collect rougher and scavenger concentrates. In tests using alkyl hydroxamate, the collector was added either to the rougher or to the scavenger, conditioned for 1-3 min. and concentrates collected as before. A dispersant such as sodium silicate or Cyquest 3223 was added prior to alkyl hydroxamate addition, depending on the type of ore and the nature and content of problem gangue.
Representative, unpulverized samples of flotation products were prepared for microscopic analysis before sending samples for chemical analyses.
Importance of Mineralogy and Assay Methods
The various copper occurrences in an ore are referred to in the industry as “Acid Soluble”, “Oxide Copper” or “Non-sulfide” copper on the basis of chemical analysis, thereby leaving no room for the possibility that much of this “Acid soluble” copper may not be present as well defined oxide minerals and that they may not even be amenable for flotation either with oxide collectors or with sulfidization-xanthate flotation.

In addition to this, the chemical assay methods used for determining such copper occurrences have not been standardized. As a result, numerous methods exist, and these methods do not necessarily give the same value for oxide copper content of an ore (often these numbers could be misleading). [A discussion of the relative merits of assay methods is beyond the scope of this paper]. Information is also sometimes unavailable on which particular assay method was used by mine assay lab or an independent assay lab.
The foregoing case studies, and flotation testing on other ores (not discussed here), have provided sufficient evidence for the following important observations:
a) Well defined oxide copper minerals such as malachite, cuprite, tenorite, etc., if present in the ore, are floated by alkyl hydroxamates. This observation is consistent with information in the literature.
b) Certain copper occurrences in the ore, for example copper-containing goethite, are not amenable to flotation and they are not recovered by alkyl hydroxamate. This observation has been ignored in past investigations. Even if species such as Cu- containing goethite were made to float, they would produce a very low grade concentrate, which may not be a desired product (direct leaching is perhaps better in such cases).
c) If AS Cu recovery obtained using hydroxamate from a given ore is poor (assuming that appropriate test conditions were used), this does not point to any deficiency in the collector properties of alkyl hydroxamate, but certainly points to mineralogical problems.
d) A microscopical examination, verified by microprobe work, is warranted before embarking on any flotation testing program. Relying solely on chemical assays of AS Cu will lead to erroneous conclusions and will prevent a meaningful cost- benefit assessment of AS Cu recovery by flotation.
Due to similar reflective light microscopy characteristics, goethite and Cu-bearing goethite can easily be misidentified as cuprite by the untrained eye. Cu-bearing goethite will also report as acid soluble copper in chemical analyses. Misidentification of Cu-bearing goethite as cuprite will lead to the erroneous conclusion that cuprite is not recovered by alkyl hydroxamates.
It would appear that most copper ores containing AS Cu also contain Cu-bearing goethite. EDX probing of a goethite particle is an accurate method of quickly identifying copper contained in the goethite lattice.
