Table of Contents
During the period 1960-65 sulfur consumption in the United States rose 40 percent to 7.96 million long tons. Since 1963, while demand has exceeded production, withdrawals from producer stocks have met the deficit. An assured steady supply of sulfur is essential for the national interest. Its applications are so diverse that the quantity used is an accurate measure of a nation’s standard of living. A severe or prolonged shortage of sulfur could adversely affect not only a nation’s strength but also its continued growth in an expanding economy.
Such materials as anhydrite, gypsum, sea water, and hydrous calcium sulfate, a byproduct of the fertilizer industry, are potential sources of almost unlimited quantities of sulfur. Anhydrite and gypsum are being used in limited quantities in the United Kingdom and other European countries to make sulfuric acid and cement clinker. This is practical only when limestone and other necessary raw materials for conventional cement manufacture are not available locally. Cheap energy is also essential; the cost of energy has prohibited wider use of this recovery method. If these sulfur sources could be converted biologically to hydrogen sulfide and then converted chemically or biologically to elemental sulfur, these materials might become available on a more competitive basis.
The Bureau of Mines is presently engaged in a number of projects dealing with the recovery of elemental sulfur by chemical methods from smelter and flue gases as well as gypsum. Considerable interest in the microbial production of elemental sulfur from gypsum was stimulated by the British in the early and mid-1950’s, after they had discovered that a microbiological process was responsible for the deposition of elemental sulfur on the bottom of several lakes located 20 miles southwest of El Agheila, Libya. Since all of the above-mentioned sources of sulfur are amenable to microbial attack with the subsequent recovery of sulfur, the microbial research herein reported supplements the current Bureau work by offering still another alternate method for recovering sulfur from natural occurring minerals and waste gases.
The objectives of this research are to develop operating techniques and to define some of the operating parameters. The main thrust of this research was directed toward the maximum microbial production of hydrogen sulfide from hydrous calcium sulfate, as chemical methods for converting hydrogen sulfide to elemental sulfur are well-known and presently being used where the volume of hydrogen sulfide is sufficient to make the conversion economical.
Materials and Methods
Sulfate Sources
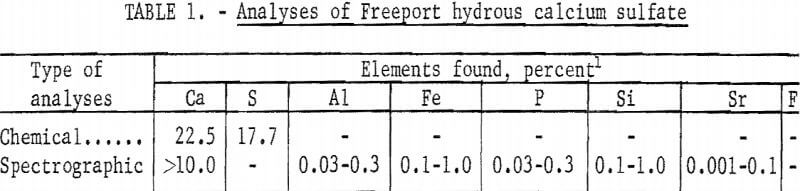
Sulfate was obtained from two sources: chemically pure hydrous calcium sulfate, and a hydrous calcium sulfate byproduct obtained from the Freeport Sulphur Company’s Uncle Sam phosphoric acid plant located northwest of New Orleans, La. The Freeport hydrous calcium sulfate was collected in a wet state and formed lumps upon drying which were broken up prior to use in various fermentors. A partial chemical analyses of the Freeport hydrous calcium sulfate is given in table 1, X-ray phase study of Freeport hydrous calcium sulfate showed the sample contained over 85 percent CaSO4·2H2O, approximately 5 percent gamma phase CaSO4, and a trace amount of 2CaSO4 · 1H2O (or CaSO4·½H2O).
Medium
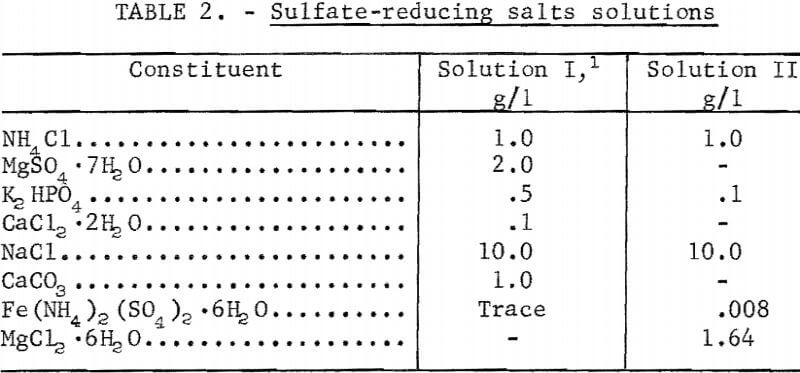
The basic salts solutions employed in sulfate-reduction studies may be found in table 2. The complete medium, as used in a fermentor, had various quantities of hydrous calcium sulfate and sodium lactate (60 percent) added to the above salts solutions. Individual constituents were varied at times, in order to determine their effect upon the rate of sulfate reduction or cell reproduction; this variance is self-evident in the various curves and tables throughout this report.
Bacteria
Two strains of sulfate-reducing bacteria were employed in these studies and are differentiated primarily on the basis of their source. The first strain used was obtained from an anaerobic digestor at the Pleasant Hills Sewage Treatment Plant, located near the Bruceton Coal Research Center, Bruceton, Pa, The second strain was obtained from the American Type Culture Collection (ATCC), located in Rockville, Md., and was designated Desulfovibrio desulfuricans ATCC 7757.
These bacteria are slightly curved rods of variable length, usually occurring singly or sometimes in short chains. They are gram-negative, actively motile by a polar flagellum, and measure 0.5 to 1.0 micron in width and 1 to 5 microns long. They are strict anaerobes which reduce sulfates to hydrogen sulfide utilizing, in most instances, an organic compound as a hydrogen donor and a source of carbon for cell structure, A typical reaction that this organism brings about may be found in the equation below:
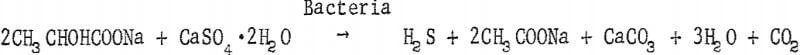
Flask Studies
Studies were conducted in 65-ml glass stoppered flasks charged with 1 ml of an actively growing culture of sulfate-reducing bacteria (usually obtained from an active fermentor) and enough of salts solution I or II to completely fill the flask once hydrous calcium sulfate and sodium lactate or other carbon sources had been added. The flasks and their contents were then incubated at 30° C for 1 week, at which time they were removed and the hydrogen sulfide produced determined using iodometric methods. Cell counts were made with a Petroff-Hauser counting chamber.
Fermentor Studies
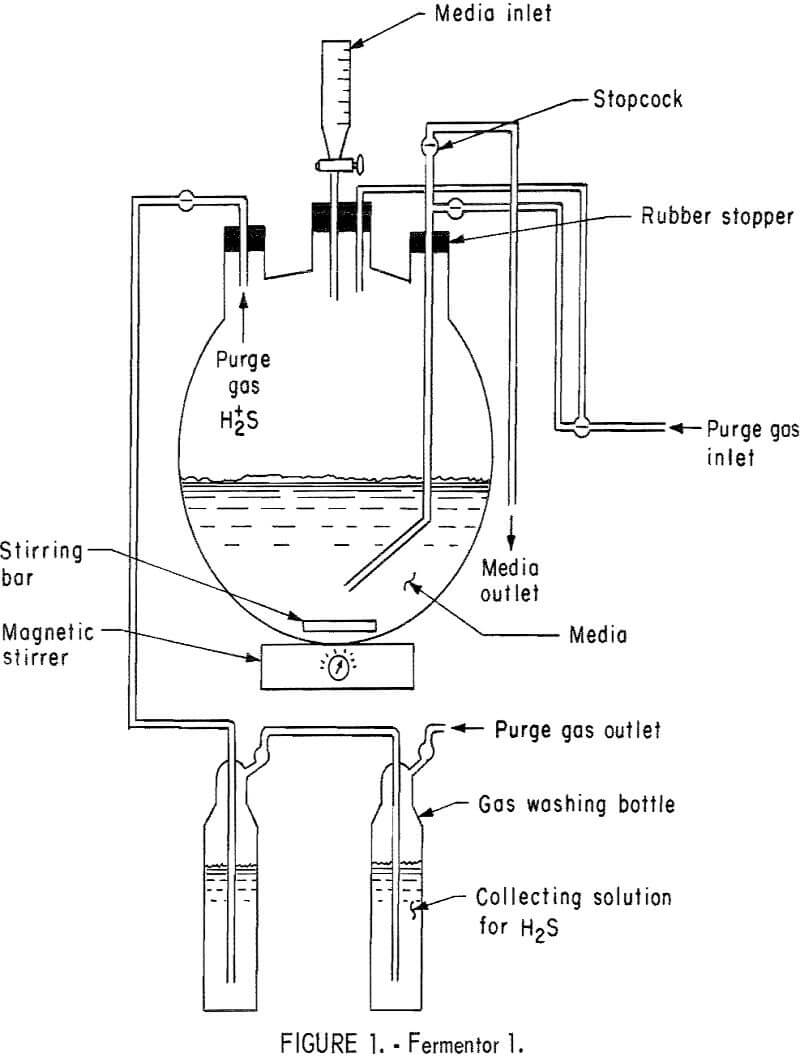
The basic design of two of the three fermentors employed in these studies are shown in figures 1 and 2. Fermentor 1, as shown in figure 1, was used in studying medium exchange rates. It was charged with 1,950 ml of solution I containing 17.5 g/l hydrous calcium sulfate, 21 g/l sodium lactate (60 percent), and was inoculated with 50 ml of sulfate-reducing bacteria obtained from the Pleasant Hills Sewage Treatment Plant. Nitrogen gas was employed as the sweeping gas for hydrogen sulfide and was fed into the bottom of the fermentor at a rate of 24 ml/hr. This fermentor was operated at approximately 30° C. Stirring was accomplished with a magnetic stirring bar and was just fast enough to keep the hydrous calcium sulfate suspended.
Fermentor 2 was a Model F-14 fermentor designed and built by the New Brunswick Scientific Co. The 14-1 jar was charged with 4,890 ml of solution I containing 17.5 g/l hydrous calcium sulfate, 21 g/1 sodium lactate (60 percent), and inoculated with 110 ml of the sulfate -reducing bacteria, D. desulfuricans. Nitrogen gas was passed through the system at a rate of 25 ml/hr while the temperature and stirring rate were controlled at 32° C ±0.25° C and 200 rpm, respectively. Through various manipulations to the medium, stirring rate, and gas flow, the system resolved itself into using solution II (hydrous calcium sulfate and sodium lactate concentrations were varied during the course of the investigation), a stirring rate of 100 rpm (varied at times), and a gas sweeping rate, across the top of the medium, of 60 ml/hr (also varied at times). A 70-percent daily exchange rate of the medium was maintained during most of the testing period. Attempts were made to maintain the pH of the fermentor solution between 7.0 and 8.0.
By changing the settings of the various stopcocks or pinch clamps associated with the above two fermentors, the direction in which the purge gas was flowing could be changed so that instead of the purge gas bubbling through the medium, it created an over pressure which was used to force the medium out of the reaction chamber and into a collection vessel.
Fermentor 3 (fig. 2) was designed and built by the authors to incorporate various factors which had become evident during the early experimentation. These factors were the use of a sand-hydrous calcium sulfate bed instead of a hydrous calcium sulfate slurry and the substitution of hydrogen for nitrogen as the sweeping gas. The bed consisted of 200 g of Ottawa sand placed on top of glass wool and supported by a perforated stainless steel plate, to which were added 50 g of Freeport hydrous calcium sulfate. The percolator was then charged with 400 ml of solution II, 40 g of sodium lactate (60 percent), and inoculated with 100 ml of effluent from fermentor 2. Hydrogen gas was used to percolate the solution and to strip the hydrogen sulfide gas from solution. Daily medium exchange rates of 500 ml were maintained, with amounts of hydrous calcium su i fate and sodium lactate (60 percent) being replaced based on their calculated consumption. Evidently the sand-hydrous calcium sulfate bed
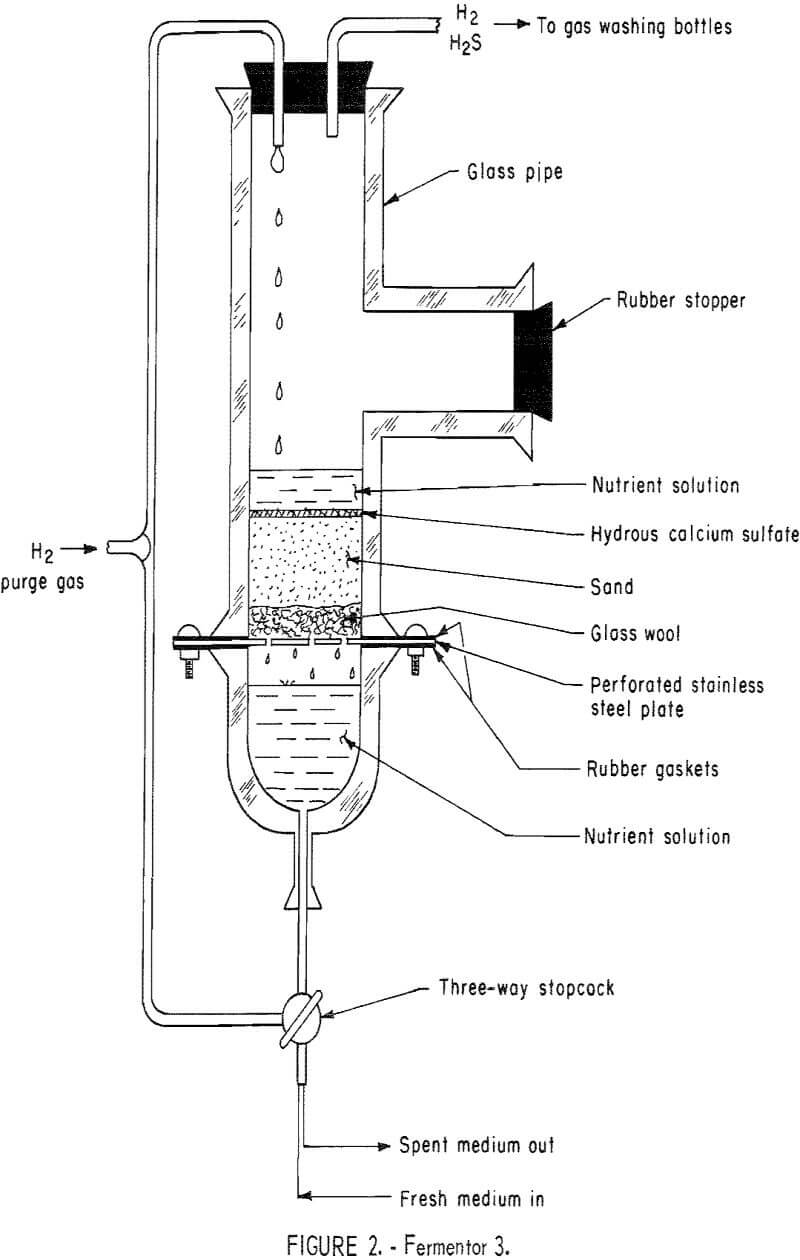
maintained sufficient numbers of sulfate-reducing bacteria to hold the system in an exponential growth phase and thereby insure good hydrogen sulfide production. The fermentor solution’s pH was maintained within a range of 7.0 to 8.0 through the daily addition of sulfuric acid. The system was operated at room temperature.
The three fermentor systems described above employed gas washing bottles charged with either a 4- to 8-percent zinc acetate or a 0.5, 1.0, or 1.5 N sodium hydroxide solution to remove the hydrogen sulfide being generated within and being swept out of the systems by either nitrogen or hydrogen gas. Hydrogen sulfide was determined by iodometric titrations of the effluent medium and the solutions obtained from the gas washing bottles.
Results and Discussion
Effect of pH
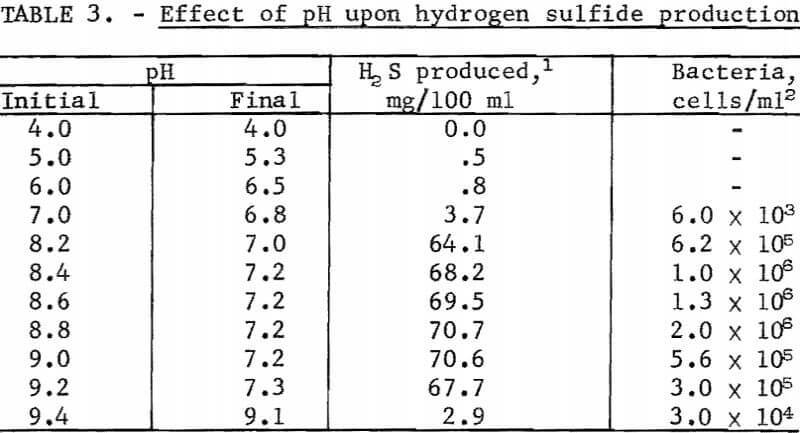
Flask studies were run to determine the effect pH has upon the microbial production of hydrogen sulfide. The results of this test are summarized in table 3. The data shows that the optimum pH range for maximum hydrogen sulfide and cell production was 7.2 to 8.8. Significant reductions in hydrogen sulfide production as well as in the number of bacterial cells generated became evident, when the medium’s pH remained below 7.0 or above 9.0.
Effect of Temperature
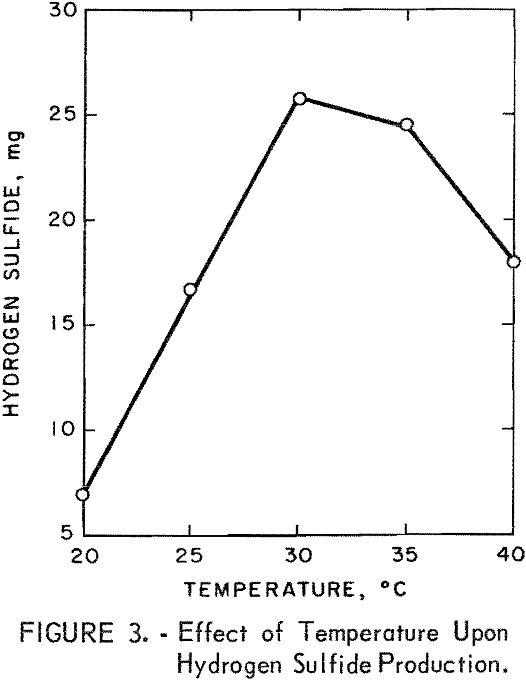
Flask studies were run to determine the effect of various temperatures upon the microbial production of hydrogen sulfide. The results given in figure 3, show that the optimum temperature range for maximum production of hydrogen sulfide was 30° to 35° C. It is to be noted that there are thermophilic sulfate-reducing bacteria which grow within a temperature range of 30° to 65° C and have an optimum growth temperature of 55° C, thus indicating the versatility of the sulfate-reducing bacteria in regards to their temperature requirements.
Carbon Source Study
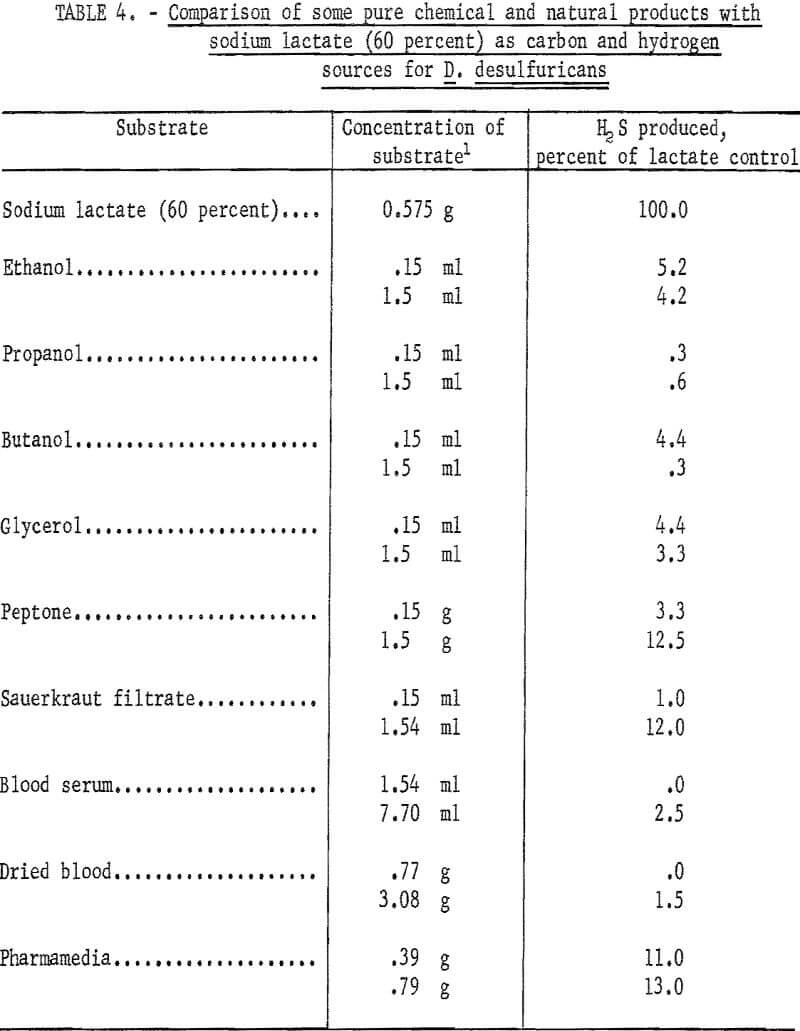
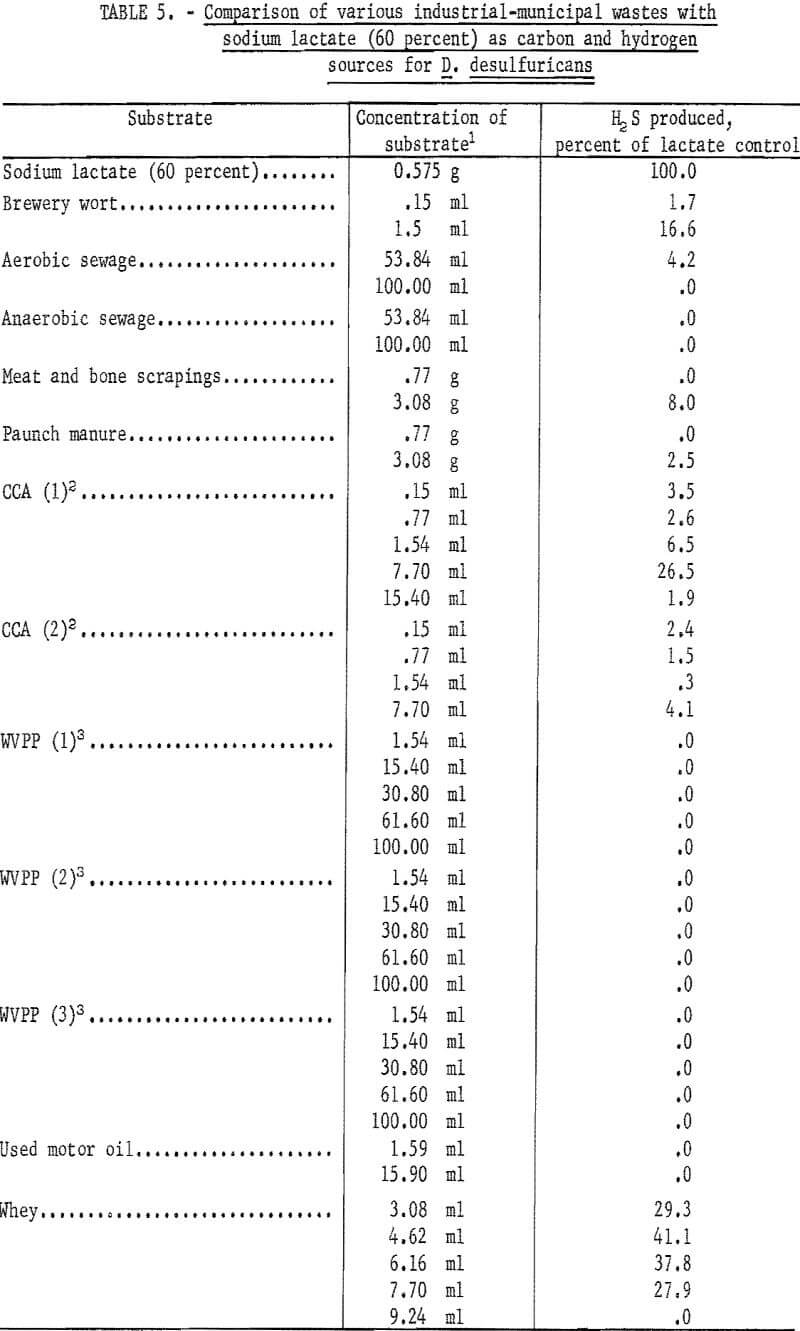
Various substrates were tested for their ability to serve as carbon and hydrogen sources for the sulfate-reducing bacterium D. desulfuricans. The materials are broken down into two groups; namely, pure chemicals and natural products, and municipal-industrial wastes. Of the pure chemical and natural products recorded in table 4 only peptone, Pharmamedia (a commercial preparation), and sauerkraut filtrate showed any significance in serving as carbon and hydrogen sources for D. desulfuricans during the reduction of hydrous calcium sulfate. However, the best of these, Pharmamedia, was only 13 percent as good as sodium lactate. Of the municipal-industrial wastes tested and summarized in table 5, whey was 41.1 percent as effective as sodium lactate, and a waste sample from the Celanese Corporation of America (CCA-1) located in Cumberland, Md., was 26.5 percent as effective as sodium lactate. The Celanese waste (CCA-1) contained acetic acid, magnesium sulfate, and other products of a cellulose degradation. No microbial response to waste samples (WVPP-1, 2, and 3) taken at the West Virginia Pulp and Paper Co. of Luke, Md., was obtained.
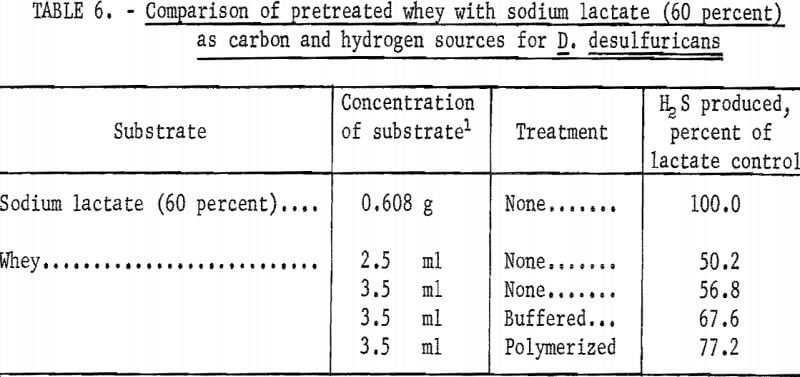
Because of the good microbial response to whey, its availability, and ease of handling, further studies were undertaken to determine if whey could be made more attractive through increased utilization by D. desulfuricans. Whey was buffered and polymerized in an attempt to increase its utilization. The results of the treatments are summarized in table 6. The polymerized whey (neutralized with sodium hydroxide and heated to boiling) showed the best response in that it was 77.2 percent as effective as sodium lactate in serving as a carbon and hydrogen source for D. desulfuricans. However, this was to be expected as the polymerization resulted in converting the lactic acid present in whey to sodium lactate and therefore had sufficient lactic acid been present in the whey, it is likely that it would have been 100 percent as effective as the chemically pure sodium lactate. The buffered whey showed a very good response in that it was 67.6 percent as effective as sodium lactate. It is felt that whey would present a very good substitute for sodium lactate, and that with additional research whey might equal or possibly exceed sodium lactate in its ability to support the production of hydrogen sulfide.
Medium Exchange Rate
In order to produce significant quantities of hydrogen sulfide for extended periods of time, a more or less continuous operation was needed in which the sulfate-reducing bacteria could be kept in a logarithmic growth phase. The apparatus best suited for this is a continuous culturing apparatus also known as a chemostat.
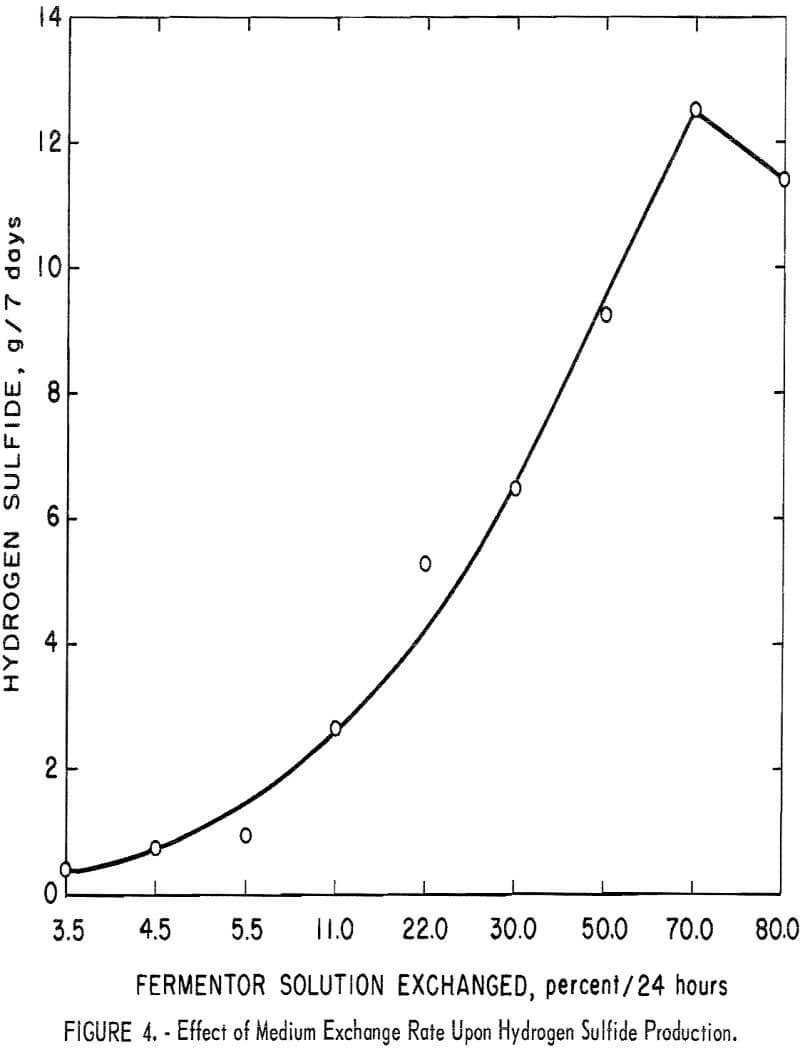
In a chemostat the waste products generated by the bacteria as they carry on their metabolic activities are continually removed by exchanging the spent medium with fresh medium. Media removal causes the loss of large numbers of viable cells and therefore must be coordinated with a cell’s generation time in order to preserve the cell population. A series of media exchange rate studies were performed in fermentor 1. Based on the data shown in figure 4, the optimum medium exchange rate for maximum production of hydrogen sulfide would be 70 percent of the solution volume per 24 hours.
Effect of Sweeping Gas Upon Hydrogen Sulfide Production
Two semicontinuous anaerobic fermentors were operated with one being continuously purged with a stream of nitrogen gas while the other was not. The fermentor which was continually purged with nitrogen gas showed a 1.7-fold increase in hydrogen sulfide produced over the system in which no purging gas was employed. This increase was believed to be due to the purge gas lowering the concentration of hydrogen sulfide in the fermentor and thereby lessening any toxic effect high concentrations of hydrogen sulfide would have upon the activity of these sulfate-reducing bacteria. Postgate has shown that sulfide inhibition does occur.
Effect of Hydrous Calcium Sulfate Solubility Upon Hydrogen Sulfide Production
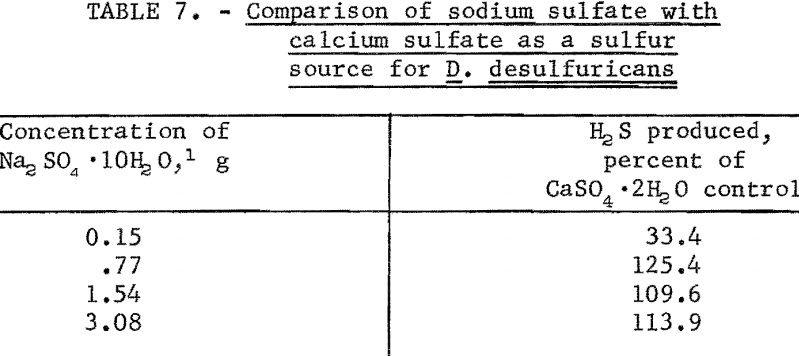
In order to test the effect of hydrous calcium sulfate solubility upon hydrogen sulfide production, flask studies were run in which a soluble form of
sulfate (Na2SO4·10H2O) was compared to hydrous calcium sulfate. The data in table 7 shows that in the proper concentrations, soluble sulfate (sodium sulfate) is utilized much more readily than insoluble sulfate (hydrous calcium sulfate), and the use of soluble sulfate resulted in an approximate 1.3-fold increase in hydrogen sulfide production over similar flasks in which the relatively insoluble hydrous calcium sulfate was used. The solubility of hydrous calcium sulfate will undoubtedly play an important role in determining the maximum rate of converting hydrous calcium sulfate to hydrogen sulfide microbially.
In conjunction with this study, tests were run in flasks to determine if a hydrous calcium sulfate byproduct of the phosphate fertilizer industry could be efficiently utilized to produce hydrogen sulfide and thereby rule out the possibility that toxic contaminants might be present in this byproduct that would inhibit the bacteria. Preliminary screening tests with hydrous calcium sulfate residues from fertilizer production have indicated that the hydrous calcium sulfate contained in this material could be converted to hydrogen sulfide at about the same rate as chemically pure hydrous calcium sulfate. This finding was supported by later experiments in which hydrogen sulfide was produced in a percolator-fermentor charged with this byproduct.
Fermentor Design
Fermentor design will play an important role in obtaining maximum hydrogen sulfide production. Such things as ease of operation, maintenance of anaerobic conditions during experimentation, durability over extended periods of time, and simplicity of design so that startups and shutdowns can be accomplished with a minimum of effort, as well as the monitoring and control of various aspects of the system such as temperature, pH, stirring, nutrient addition and medium exchange, will all effect hydrogen sulfide production.
The experimentation conducted with different fermentation systems during the course of this investigation has led from the initial fermentor (fig. 1) through a commercial fermentor, New Brunswick Model No, F-14, to the final fermentor (fig. 2). Studies conducted in these three fermentors coupled with flask studies, have led to many manipulations to the fermentors in a search for increased hydrogen sulfide production. Through these studies it was possible to increase the hydrogen sulfide production by a factor of 238, or from 0.03 g H2S/l of fermentor volume per 24 hours to a high of 7.13 g H2S/l of fermentor volume per 24 hours at the time the project was terminated. It is felt that the true potential of the percolator-fermentor was never achieved as the sodium lactate (60 percent) level in the nutrient salts solution II had only reached 14 percent (w/v). The research reported herein has led the authors to believe that the technical feasibility of producing hydrogen sulfide from hydrous calcium sulfate with sulfate-reducing bacteria has been fully demonstrated and that credence has been given to the ultimate possibility of economically converting vast gypsum deposits into elemental sulfur.
Conclusions
The data presented support the following conclusions:
- The optimum pH and temperature ranges for maximum hydrogen sulfide production were 7.2 to 8.8 and 30° to 35° C, respectively,
- Of all compounds tested as a substitute for sodium lactate (60 percent), only whey gave significant yields of hydrogen sulfide. It was found to be between 50 and 77 percent as effective as sodium lactate in serving as a carbon and hydrogen source for the sulfate-reducing bacteria.
- The optimum medium exchange rate to be employed in the operation of a hydrogen sulfide fermentor was determined to be 70 percent of the fermentor’s solution volume per 24 hours.
- The use of a purge gas to remove hydrogen sulfide from a fermentor was found to increase hydrogen sulfide production by 1.7 times.
- The relative insolubility of hydrous calcium sulfate was determined to be one of the limiting factors in producing hydrogen sulfide.
- Hydrous calcium sulfate produced as a waste byproduct from one phosphate fertilizer plant was shown to be converted to hydrogen sulfide at about the same rate as chemically pure hydrous calcium sulfate.
- Fermentor design was determined to be important to the production of hydrogen sulfide with a percolator style design giving maximum yields of hydrogen sulfide.
- Maximum hydrogen sulfide production obtained was 7.13 g H2S/l of fermentor volume per 24 hours.
- Experimental data indicate that hydrous calcium sulfate can be microbially converted to hydrogen sulfide at a much faster rate than the 7.13 g H2S/l of fermentor volume per 24 hours by increasing the sodium lactate content of the fermentor.
