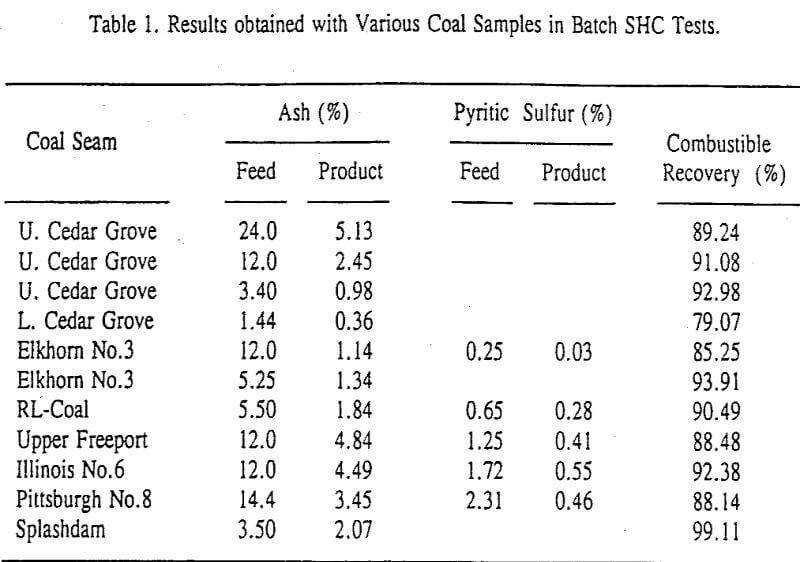
A novel ultrafine coal cleaning process has been developed at Virginia Tech that is capable of producing superclean products. Compared to other selective aggregation processes, such as oil agglomeration and selective flocculation, this process relies on the natural hydrophobicity of the coal surface to induce the selective coagulation of coal particulates in an aqueous suspension. Since the associated mineral matter is dispersed by simple pH control, a pH modifier is the only chemical requirement for the process.
Experimental
A sample of run-of-mine Elkhorn No.3 seam coal, Kentucky, was obtained from Consolidated Coal Company to study the effect of the various process parameters. The ash and sulfur contents of the raw coal sample were 12.0% and 0.81%, respectively. Samples were also obtained from a number of different coal seams to test the feasibility of the process. As soon as each sample was received, it was crushed to -6 mm using a laboratory jaw crusher. The crushed coal was then split into representative lots of 1000 grams each, placed into air-tight containers, and stored in a freezer at -20°C to minimize oxidation.
In order to determine the effect of pH on the stability of a suspension containing both coal and mineral particles, tests were conducted over a pH range of 3-11 on a run-of-mine Elkhorn No.3 coal. The results show that both coal and mineral matter coagulated between the pH values of 3 and 8. The coagulation of coal continued up to a pH value of 9, while the mineral matter began to be dispersed.
The Elkhorn No.3 coal samples were ground in an attrition mill for varying lengths of time and subjected to SHC experiments to study the effect of particle size. The mean volume particle size (d50) of each sample was determined by an Elzone 80-xy particle size analyzer.
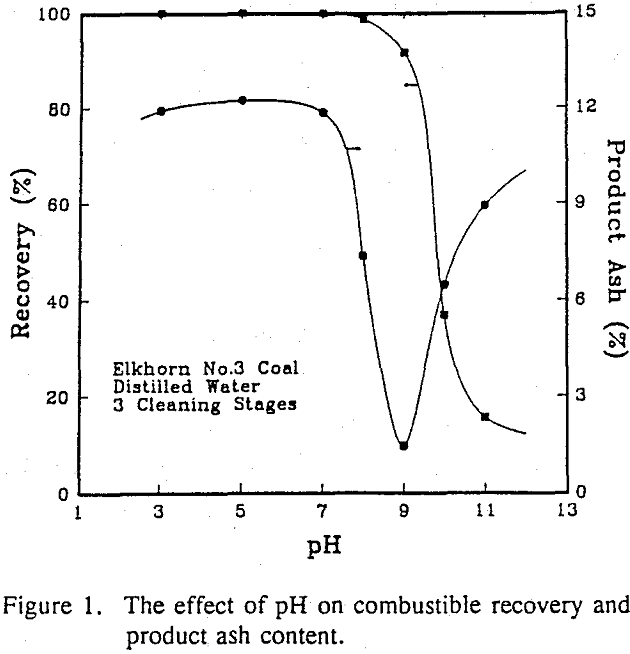
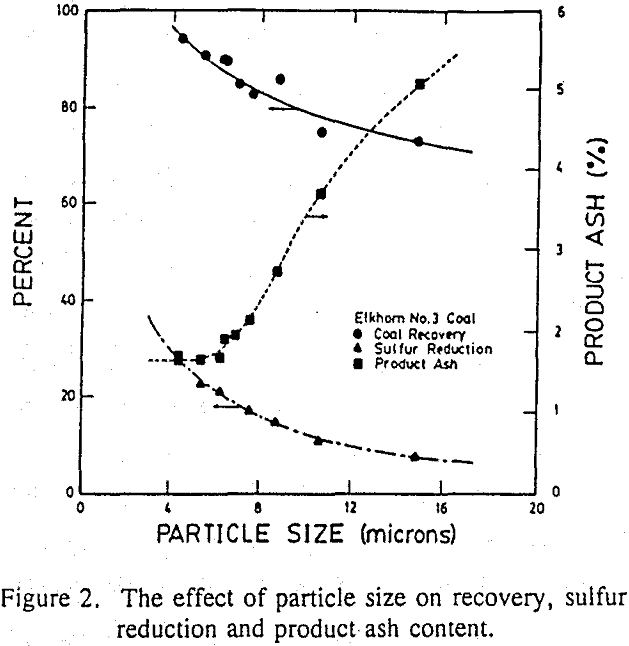
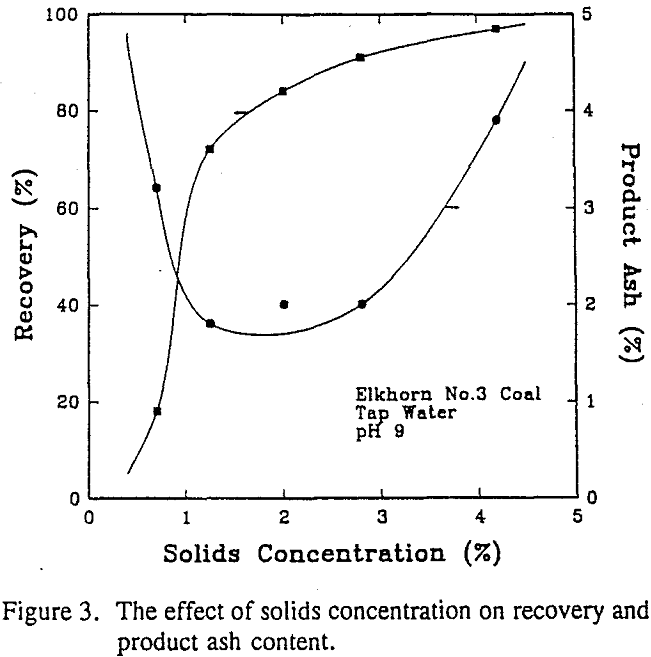
Discussion
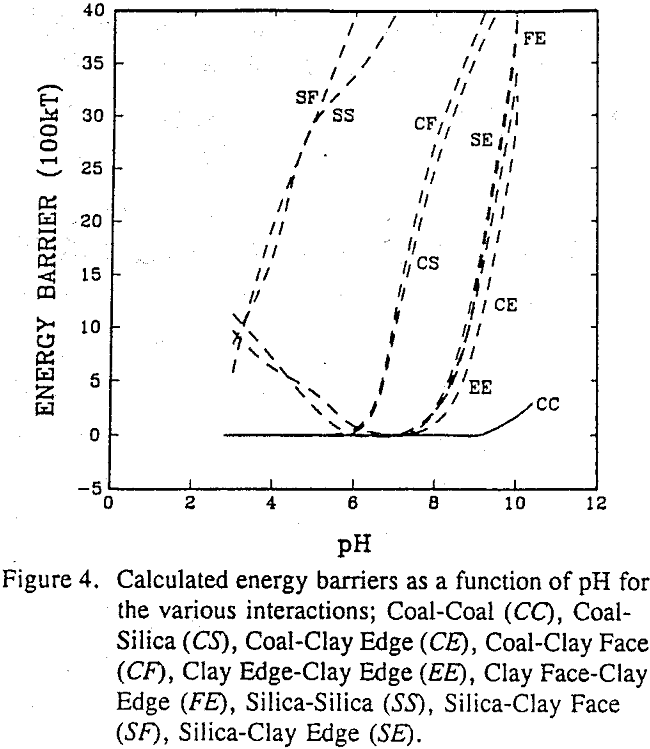
An attempt was made to employ the classical DLVO theory to describe the SHC process. According to this theory, the stability of lyophobic colloids is determined by the sum of the electrostatic repulsive energy (VR) and the London-van der Waals dispersion interaction energy (VA):
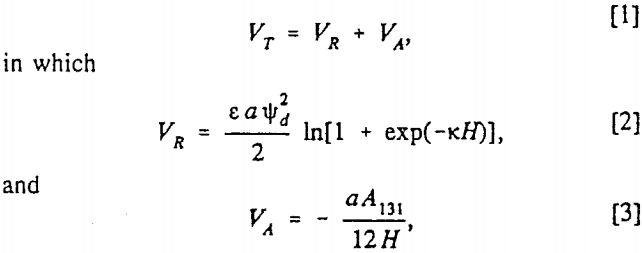
The coagulation efficiency of silica was virtually zero above pH 4, but reached 38% at pH 2 where the energy barrier was minimum. The coagulation efficiency of kaolin sharply medium, a the radius of the spheres, H the separation distance between the spheres, e the dielectric constant, ψd is the Stern potential and k the Debye reciprocal length.
The selective hydrophobic coagulation process utilizes the hydrophobic nature of coal surfaces to selectively aggregate and separate coal particles from the dispersed mineral matter. pH modifiers are the only chemical requirements for the process.

