Table of Contents
In view of the poor floatabilities of fine particles their treatment prior to flotation becomes essential and aggregation is a means for improving the recoveries. Many of the variables which govern the flotation process also influence the aggregation mechanism and may be classified into physical, chemical and geometrical. Earlier investigations report the effects of particle size, ratio of fines to coarse particles, pH, collector concentration, conditioning time, stirring rate and cell geometry on the aggregation of particles in single mineral systems and also for some ores. The collector used and the type of adsorption (physical or chemical), pH and surface coverage (degree of hydrophobicity), all contribute to an energy barrier between the particles. Aggregate formation and its strength or stablility are governed by the energy input to the system. The shear force, generated by an energy input (angular velocity expressed as stirring rate, rpm) to a suspension containing hydrophobic fines (<5-10 µm) and coarse (~ 40-50 µm) particles, enables better particle collisions and consequently adherence of the former to the latter due to hydrophobic association. The aggregates thus formed are recovered by flotation.
The technique is known by different names: carrier flotation, piggyback flotation, ultra flotation, floc flotation, autogenous carrier flotation, high intensity conditioning/flotation and shear-flocculation/flotation. However, a subtle difference exists between shear-flocculation and other aggregation methods, in that the hydrophobic fines are aggregated by high shear rates. In other techniques, either a coarse carrier mineral of the same species (autogenous carrier flotation) or a different carrier like calcite (e.g. carrier flotation for the separation of anatase impurity from kaolin clay, also known as ultra flotation, piggyback flotation) is used to recover fines. In different mineral-collector systems the stirring rates reported varied from 800-3000 rpm. A detailed description of the techniques is given elsewhere.
Theory
The electrical double layer model is shown in fig. 1. A potential difference at the interface of a solid-liquid is due to one or more of the following reasons: unequal distribution of constituent ions, ionisation of surfee groups, isomorphous substitution, specific adsorption of ions and dipole orientation.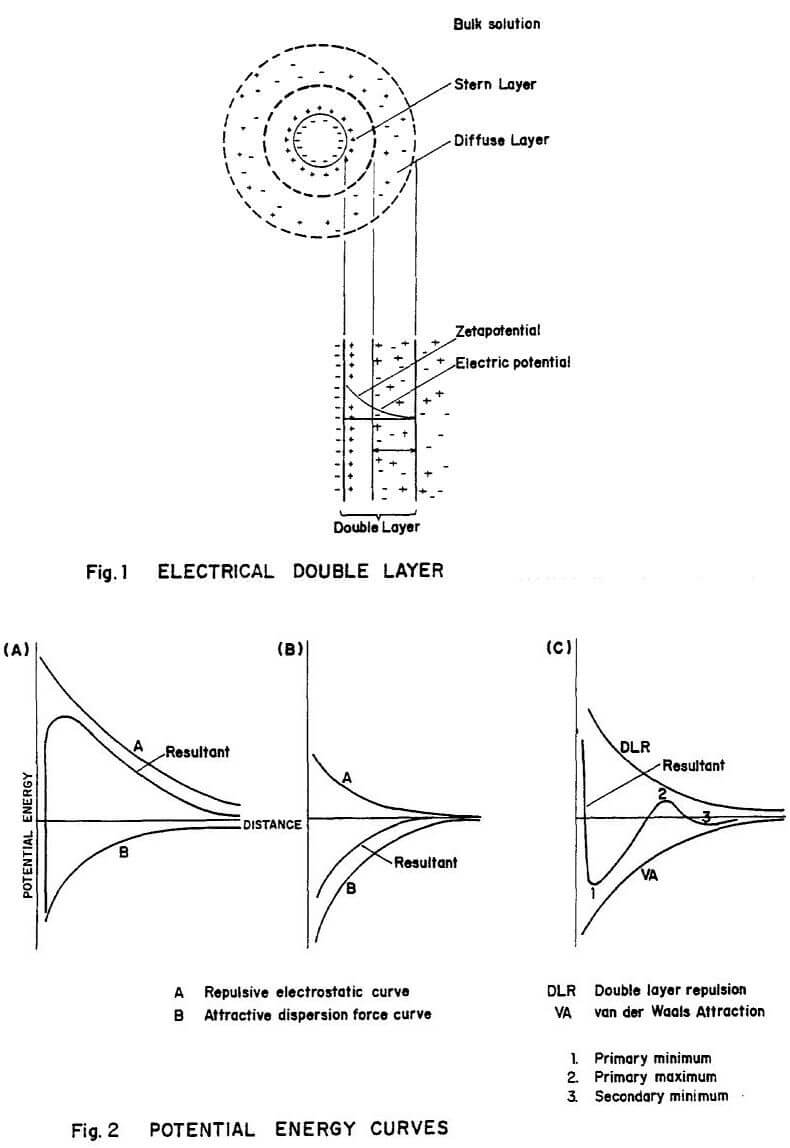
Particles holding similar charge repel due to the energy barrier between them. It is the balance between the electrostatic repulsive forces and the van der Waals attractive forces that finally governs the colloidal stability and is explained by the DLVO theory. In the potential energy-distance curves (fig. 2), a positive resultant (fig. 2a) leads to repulsion due to the energy barrier between the particles, thereby resulting in a stable colloid; whereas, a negative resultant (fig. 2b) corresponds to attraction and destabilization thus forming an unstable colloid. The resultant curve in Fig. 2c corresponds to irreversible coagulation when the net force of attraction is higher than the repulsion. For colloidal particles collisions occur because of Brownian motion; whereas, mineral particles are larger in size and the van der Waals attractive energy is not strong enough to overcome the repulsive energy.
Two hydrophobic particles holding similar charge experience the energy barrier. When sufficient energy is generated to create particle collisions, then the resulting mass would be an aggregate formed by the energy of hydrophobic association. In addition to the van der Waals attractive and electrostatic repulsive forces, a third one, the hydrophobic energy needs to be considered when the particles are hydrophobic. The total potential energy is a sum of all the three.
The hydrophobic energy, in turn, is considered to consist of (1) energy resulting from the association of hydrocarbon chains and (2) a long distance hydrophobic energy which results from the thinning of liquid film, as the water molecules surrounding two hydrophobic particles are repulsive and consequently the particles are driven closer to each other. When particles like ilmenite or magnetite are involved, magnetic attractive energy should also be considered, depending on the intensity.
Flotation of aggregates were reported to show improved recoveries of fine particles. In mixed mineral systems, particles of the same species were shear-flocculated and selectivity was achieved by chemical control. However, work on complex ores directed towards selectivity in aggregation is meagre. Despite the beneficial effect i.e. improvement in the flotation recovery of fines by aggregation, it is surprising that the investigations still remain on a laboratory scale.
Hydrophobic Agglomeration of Fine Molybdenite
Molybdenite. Six size fractions of molybdenite were prepared by classification of a molybdenite concentrate in a cyclosizer. An average particle size of these size fractions was measured with a Quantachrome Microscan Particle Size Analyzer. While all the size fractions were used in the preliminary flotation tests, only the finest two with the average size of 12 µm and 8 µm were utilized in the subsequent tests. All size fractions were purified by treating with diethyl ether to remove an organic contamination, with sulfuric acid to remove oxide layers, and with sodium cyanide to leach surface metallic impurities. The BET (N2) specific surface area for the 12 µm sample was measured to be 1.54 m²/g, while for the 8 µm sample this was found to be 3.29 m²/g.
Quartz. A high purity hand sorted sample of quartz has been used throughout. After grinding, a -60 +400 mesh size fraction was selected. The sample was purified by chemical treatment with a solution of hydrochloric acid (200 g/l) at room temperature followed by washing with distilled water.
Agglomerant. The UBC-1 latex was synthesized in the Department of Mining and Mineral Process Engineering of the University of British Columbia via emulsion polymerization. The mean particle size for this latex, determined with a Hitachi S-2300 scanning electron microscope, was found to fall in the range from 0.5 to 1 µm. Electrophoretic experiments revealed that the latex particles were negatively charged over the entire pH range. The films prepared by evaporating the latex were very hydrophobic with an advancing contact angle of 60 degrees.
Flotation Tests. A mixture of 15 g of purified molybdenite with 485 g of purified quartz was used in each flotation test. The tests were carried out in an Agitair LA-500 flotation machine equiped with a 1.5 litre cell, at 900 rpm and at an air flowrate of 7 L/min. The tests were conducted with 12 g/t of MIBC at pH 11 (NaOH). The overall flotation time was fixed at 6 min, but the consecutive concentrates were collected over 10 sec periods.
In the next series of experiments carried out with the two finest molybdenite size fractions, the latex – which had been added first – was conditioned with the pulp for 2 min and this was followed by the addition of MIBC and conditioning for 1 min more; 700 rpm was selected for conditioning.
In the subsequent flotation tests, a portion of MIBC needed in the experiment was first pre-mixed with the UBC-1 latex and this combination was then used as a flotation/agglomeration reagent.
Adsorption tests. Adsorption of MIBC onto molybdenite was studied using a total carbon apparatus to detect concentration of MIBC before and after conditioning with molybdenite.
2 g batches of the molybdenite have been conditioned in 75 ml of MIBC solution in distilled water for 2 hrs at room temperature. The experiments revealed that the MIBC concentration after 1 hr conditioning with MIBC was practically identical to the concentration after 2 hrs conditioning but the lime of 2 hrs. was selected for all the adsorption tests. Centirfugation was employed to obtain a clear supernatant which was then analyzed for a carbon content in a total carbon apparatus.
 |
 |
