During the radium boom in the early part of the twentieth century, the basic chemistry of uranium was fairly well defined. Uranium production has progressed from the status of a radium byproduct to a very important industry. With the ever increasing need for more efficient and cheaper methods of processing uranium ores, new techniques are being developed. Since uranium and vanadium often occur together in nature, the latter element will also be considered. As production rates, costs, and certain aspects of uranium chemistry are still classified by the Atomic Energy Commission, these will not be discussed.
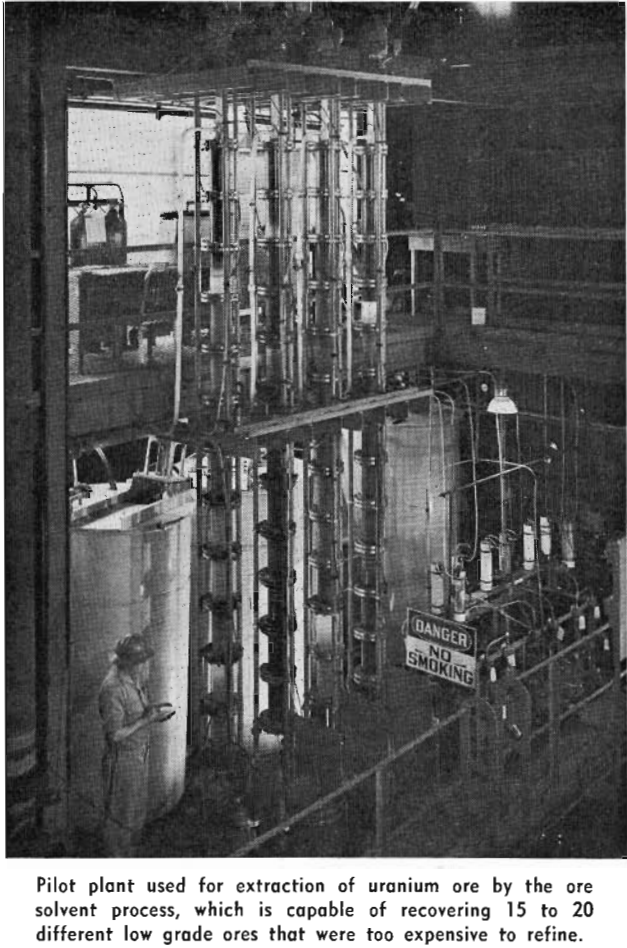
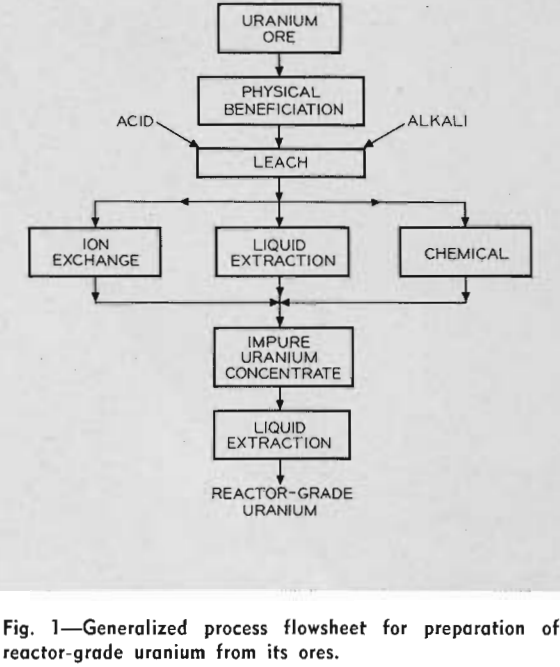
Leaching of Uranium From Ores—In general, after proper conditioning, the raw ores are treated directly. Acid leaches are employed for treatment of high silica ores, while alkaline carbonate leaches are used for high lime ores. The presence of vanadium in the deposit complicates the process, since a sodium chloride roast is required to ensure its complete dissolution in the leach liquors.
Treatment of High Silica Ores: Because silica reacts with caustic soda or soda ash, an acid digestion circuit is used for recovering uranium from high silica ores. At the present time, sulfuric acid is used almost exclusively for this purpose. In a very few cases, hydrochloric acid has been used because of the ready availability of materials for its preparation.
Recovery of the Impure Uranium Concentrate from the Leach Solutions: As the AEC specifications for the acceptable uranium oxide concentrates are on a contract basis, they vary with each producer. In general, however, the concentrates must assay greater than 75 pct U3O8 (although an acceptable 65 pct U3O8 is known) and contain only a limited amount of specified foreign elements.
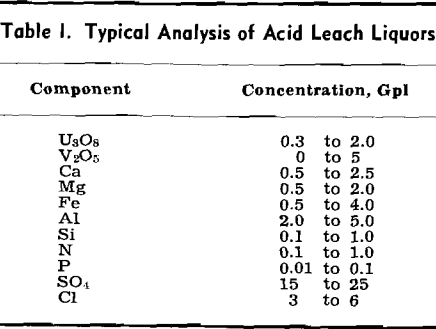
Ion Exchange—This method for producing an impure uranium concentrate has been studied in considerable detail for the past ten years. Most of the plants constructed on the Colorado Plateau during the past three years are employing ion exchange. It can be used successfully for treatment of either clarified liquors or for solutions containing solids. The latter is called the resin-in-pulp method (RIP). There are at least five domestic commercial uranium processing plants which are either under construction or in the final design state, which are using, or plan to use, the RIP process. Although the engineering of the two methods is different, the basic principles are the same. This represents the
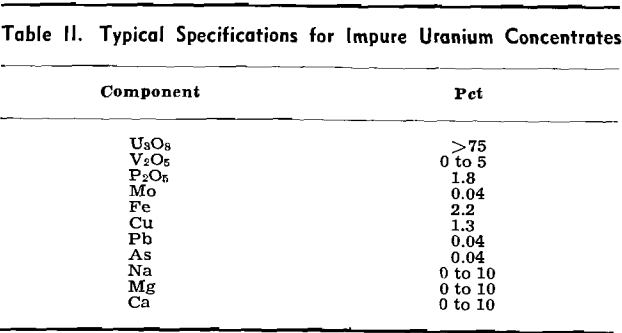
first use of ion exchange in the field of extractive hydrometallurgy.
It has been found that the amount of uranium which the resin can adsorb increases with pH. This is explained qualitatively by the fact that bisulfate has a greater affinity than sulfate for strong base anion-exchange resins. Consequently, fewer resin sites are available for uranium adsorption in strong acid solutions where the formation of bisulfate ions is favored. These leach solutions are adjusted to a pH of 1.3 to 1.7 before the ion-exchange treatment.
The ion-exchange process, in general, consists of adsorption, elution, and the recovery of uranium from the resin strip solution, or eluate.
After the ion-exchange resin is contacted with sufficient leach liquor to saturate it with respect to uranium, removal of the uranium from the resin is required. This is accomplished by treating the resin with acid solutions containing sodium or ammonium salts of chloride and/or nitrate. Uranyl sulfate complexes cannot be adsorbed from solutions which are more than 0.5 mol in chloride or nitrate ions.
Although the ion-exchange method is technically superior to the precipitation technique, certain limitations to its use exist due to the adsorption of poisons. The small amount of iron which is absorbed can be eluted before uranium.
As an example of typical production rates and scale of operation for three columns with the dimensions described previously, a 15-hr process cycle will be considered. During this time, 97,000 gal of leach liquor containing 405 lb of uranium oxide were processed. Approximately 10,000 gal of eluate were recovered containing 404 lb of uranium. The equivalent uranium oxide concentration in the eluate was 49 gpl representing a 99.8 pct recovery of the uranium.
Although the extraction of uranium and vanadium from clarified acid liquors by ion-exchange resins is successful, the RIP technique is becoming very widely employed. This method involves grinding and leaching of the ores, desanding the pulp, and selective recovery of the solubilized uranium by contacting the slime-bearing leach liquor with anion-exchange resins. The resins are contained in a wire basket of such a mesh size as to permit flow of the slimes through the screen and yet retain the resins. The resins are moved in an up and down manner in the tanks (called banks) through which the pulp flows. The pulp feed is advanced from tank to tank to give a countercurrent effect. Elution of the uranium-saturated resin is carried out in an analogous manner.
Liquid Extraction—Separation by liquid extraction requires the selective transfer of solutes between immiscible, or partially immiscible, liquid phases. This, in most cases, requires the mixing of an organic liquid having a low solubility in the aqueous phase with an aqueous solution containing a mixture of solutes. Conditions for the selective transfer of solutes are required. Since the complete separation of solutes is generally not obtained in a single contact of the two phases, a number of extractions, properly carried out, are required. Many commercial liquid extractors are available to carry out this process on a continuous basis.
In determining the utility of an organic reagent in liquid extraction, certain requirements are necessary. It is obvious that high selectivity for the desired elements is necessary. A reagent is considered selective if it results in the extraction of one element, or group of elements, to a much greater ex¬tent than for other elements in the system. Adjustment of aqueous phase conditions is necessary to obtain optimum selectivity and separation. .
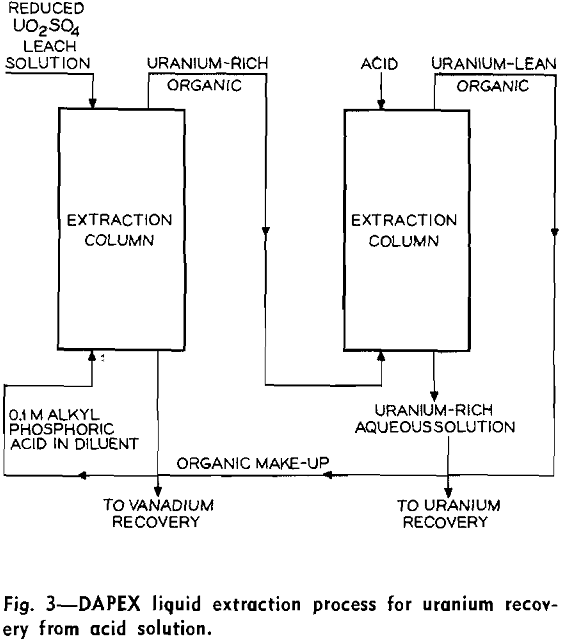
DAPEX Process—The testing of many organophosphorous compounds has been reported for extraction of uranium and vanadium from acid solutions. Both neutral and acid reagents have shown promise. These compounds include mono, di, and trialkyl phosphates, alkyl phosphoric and phosphenic acids and their esters, alkyl phosphites, phosphine oxides, and alkyl diphosphates and diphosphonates. One of the most promising of these systems entails the use of di(2-ethylhexyl) phosphoric acid as the organic extractant. Because of engineering consideration, this organic extractant is dissolved in an inert solvent, such as kerosene, prior to extraction.
In the DAPEX process, sufficient alkyl acid phosphate is generally dissolved in the carrier solvent to give a 0.1M solution. Upon contact of this dilute extractant solution with the acid leach liquor, the reaction as given in Eq. 5 occurs. R refers to the alkyloxy radical. It has been observed that the maximum selective extraction of uranium is obtained at a pH of about one. Since Fe+³ also extracts to an appreciable extent, it is necessary to reduce it to the ferrous state prior to extraction. Vanadium, which is also extracted by the acid phosphate, is transferred to a much lower extent than uranium under these conditions.
AMEX Process—Over 200 amines have been screened at the Oak Ridge National Laboratory as extractants of uranium from acid solutions. In order to meet solubility requirements, these have been limited to the long chain derivatives. Secondary and tertiary amines have been reported to be technically superior to primary amines. Chain branching also aids in uranium extraction. In order to compare the extractability by some of these amines, several distribution coefficients of uranium, Kv. The distribution coefficient for uranium is defined as the ratio of the metal concentration in the organic phase to that in the aqueous phase at equilibrium.
Treatment of Alkaline Leach Liquors—The recovery of uranium from alkaline leach solutions is somewhat simpler than from the acid leach liquors because fewer impurities are solubilized during the carbonate treatment of the ore. In general, a change in pH is employed to effect the precipitation of the uranium. This entails the precipitation of either sodium uranyl vanadate or sodium polyuranates.
In the former method, sodium carbonate solutions of uranium and vanadium are neutralized with sulfuric acid to a pH of from 3 to 5. As the carbon dioxide is boiled off, a quantitative precipitation of uranium as sodium uranyl vanadate is obtained. This solid, called yellow cake, is further refined to form U3O8.
Another method of recovering uranium from these liquors entails the addition of hydrogen under pressure in the presence of powdered nickel to the alkaline solutions at 150°C. The sodium uranyl tricarbonate is reduced to uranium oxide. After filtering of the uranium oxide, the filtrate is recycled to the circuit.

