A Survey of the Japanese chemical industry in 1949 revealed, among other things, that sulphur-bearing blast furnace gas from the Kosaka smelter of The Dowa Mining Co. Ltd., Kosaka, Japan, was being exhausted to the atmosphere as waste.
This evaluation revealed a relatively new type of roaster, the Dorrco FluoSolids reactor, which was reported to be capable of roasting the copper sulphide concentrate in an excess of air with the production of an SO2 gas strong enough for acid production and a water-soluble copper sulphate calcine. The copper sulphate then could be leached from the roasted ore and electrolytic copper recovered from the leach solution in much the same manner as practiced by the Chile Exploration Co. for treating naturally occurring copper sulphate ores. Use of a reactor of this type would eliminate the need for a blast furnace and converter and, in addition, would effect savings in labor and fuel.
The Dorr Co., sponsors of fluidization technique in the metallurgical field, suggested that sulphate roasting be tested out on a laboratory scale and that, if successful, a commercial Dorrco FluoSolids system be constructed.
Testing Program
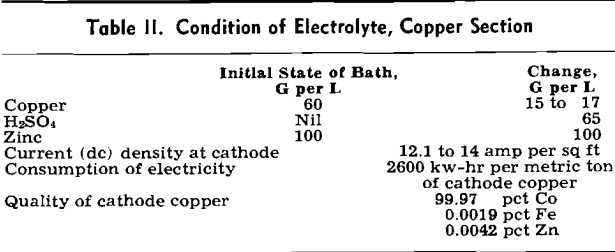
In February 1951, Mr. Tsunoda went to the United States to witness the application of the FluoSolids system to sulphate roasting at The Dorr Co.’s laboratory. The flotation concentrates tested analyzed 8.7 pct Cu, 15.4 pct Zn, 21.6 pct Fe, 2.93 pct Pb, 32.7 pct S, and 11.3 pct insoluble. These concentrates were 80.3 pct —200 mesh.
Laboratory tests indicated that copper and zinc of high solubility were present in the calcines produced in the FluoSolids reactor for subsequent leaching.
The conclusions drawn from these tests were: 1—95 pct of the copper is converted to copper sulphate, 2—90 pct of the zinc is converted to zinc sulphate, 3—5 pct of the iron is converted to ferrous and ferric sulphate, and 4—a suitable roasting temperature is 700°C.
Plant Flowsheet
The flotation concentrates are produced at the Hanaoka mine, which is situated about 24 miles south of Kosaka. The mill and smelter are connected by a railroad having two different track gages. Flotation concentrates are placed in steel boxes of two-ton capacity and loaded onto standard gage flat cars at the mill. These boxes are transferred to flat cars of the narrow gage railroad at the junction point of the two systems by a crane. On reaching the smelter, the concentrate is dumped into the storage bin.
The Dorrco FluoSolids reactor, 20 ft ID by 16 ft high, roasts the concentrates at 700°C, and an automatic control holds this temperature within ±5°C. Roaster gas, containing fine dust, passes to a waste heat boiler for heat recovery and then to a two-stage cyclone and Venturi scrubber system for recovery of the fine dust. Calcine product weight distribution is as follows: reactor over flow, 48 pct; waste heat boiler, 19 pct; cyclones, 28 pct; Venturi scrubber, 4.75 pct; and stack loss, 0.25 pct.
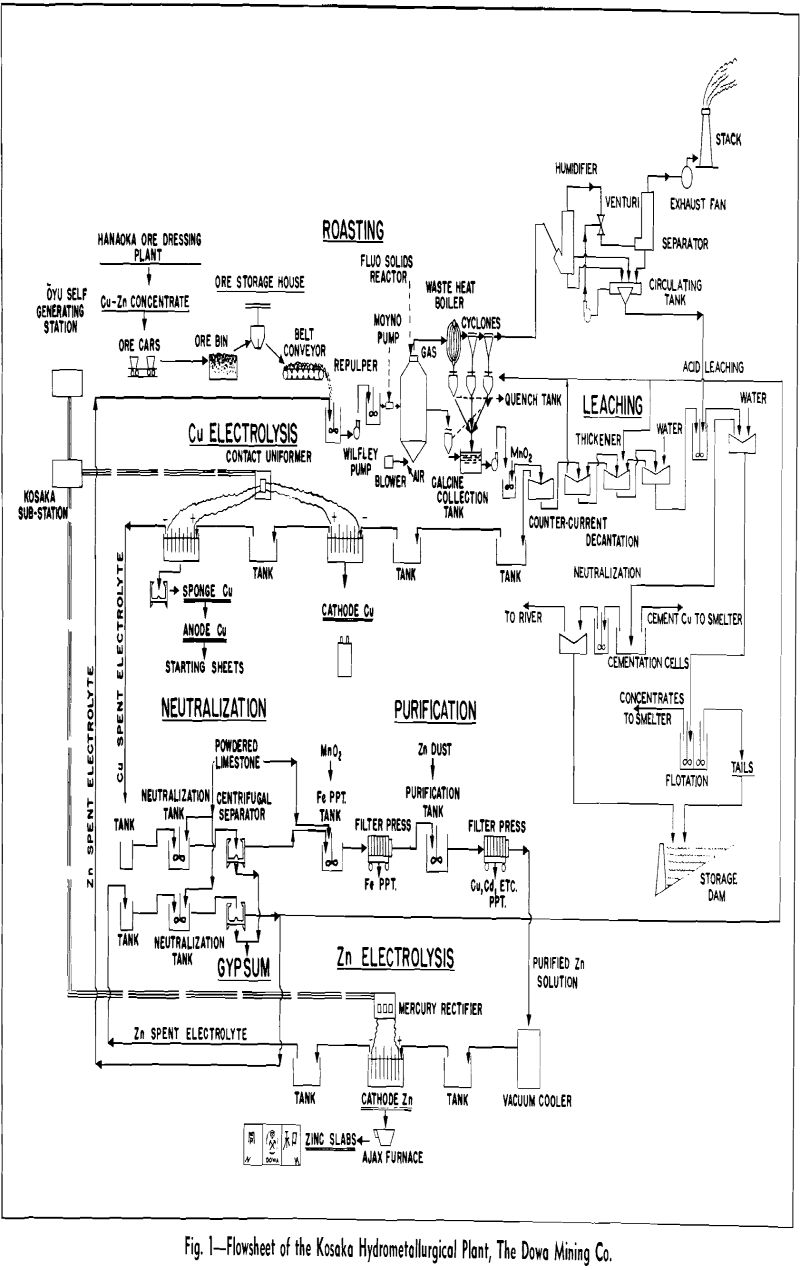
Two separate leach circuits are used. In the primary circuit, solids collected from the reactor overflow, waste heat boiler hoppers, and the cyclones, amounting to 95 pct of the total solids, are given a 5 hr leach at 70°C in a series of three wooden agitators equipped with acid-proof mechanisms.
Solution and residue in the main circuit are separated in a four-stage Dorr countercurrent decantation system. As an aid to washing, some of the neutralized solution from the zinc tank house, containing 27 g per 1 Zn, is added to the second-stage thickener and fresh water is added to the fourth stage. The solution from the primary thickener is sent to the copper tank house after clarification at the following analysis: 54 g per 1 Cu, 100 g per 1 Zn, and 2 g per 1 Fe.
Residue from the final thickener of the countercurrent decantation circuit is pumped to the secondary leaching tank where it joins the pulp from the scrubber. The solution from the single-stage washing thickener used in this circuit is sent to iron cementation cells for recovery of the copper. The solution from the cementation cells is then neutralized and discharged into the river. The cement copper recovered in this circuit is refined in the smelter.
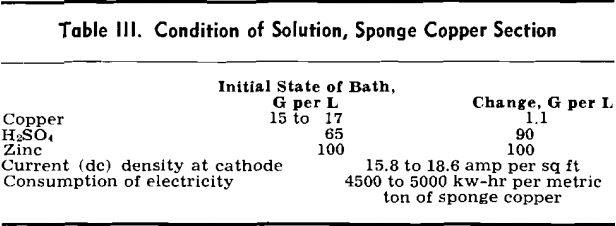
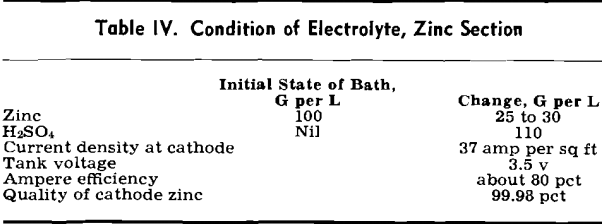
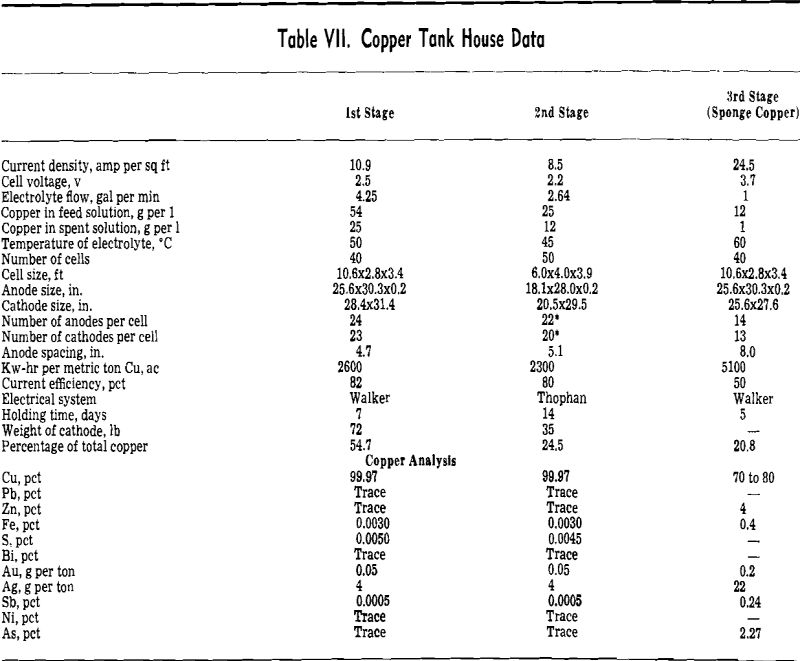
Electrolysis of Zinc: Zinc electrolysis is carried out at 36° to 38°C with insoluble lead anodes containing 1 pct Ag. Solution enters the zinc tank house at 100 to 110 g per 1 Zn at 20°C. The fresh solution is run into a circulation tank and mixed with cell solution to give the necessary acid strength.

