Table of Contents
- Experimental Procedure
- Acid Pretreatment
- Precipitation of Silver
- Aqua Regia Dissolution of Gold
- Precipitation of Metallic Gold
- Refining of Precipitated Gold
- Cyanidation of Acid Leached Residues
- Results and Discussion
- Acid Pretreatment
- Precipitation of Silver
- Aqua Regia Dissolution of Gold
- Precipitation of Metallic Gold
- Refining of Precipitated Gold
- for Recovery of Residual Precious Metals
- Slurry Solids for Acid Leaching Procedures
- Recommended Procedure for Zinc Precipitates
- Recommended Procedure for Steel Wool Cathodes
- Handling and Disposal of Acid Solutions and Acid Wastes
- Zinc Precipitates & Steel Wool Cathodes by
We investigated chemical methods for producing high-purity gold from precious-metal-bearing zinc precipitates and steel wool cathodes. Precious-metal-bearing zinc precipitates and steel wool cathodes are unrefined products from conventional cyanidation and heap leaching-cyanidation operations. The zinc precipitates contained 14.40 pct Au and 0.35 pct Ag. The precious-metal-bearing steel wool cathodes contained 20.65 pct Au and 4.84 pct Ag. The precipitates and cathodes were treated with dilute acid to solubilize the silver and/or base metals. The gold-bearing residue was leached in dilute aqua regia to solubilize the gold. High-purity gold was precipitated from the aqua regia solution with oxalic acid, sulfurous acid, sodium bisulfite, and gaseous sulfur dioxide. The leaching-precipitation experiments recovered 99.9 pct of the gold. The gold precipitates ranged in fineness from 997 to 999 fine.
The chemical refining method provides a viable technique for the smaller operator to produce high-purity gold without using pyrometallurgical refining methods.
The increase in precious metals prices since 1975 has stimulated extensive exploration programs. Concurrently, the Bureau of Mines has devised improved leaching technology for processing gold-silver ores. Many of the precious metal resources are too small to warrant expenditure of large amounts of capital to recover the contained values. The operators of small properties are limited in processing technology to low-cost heap leaching or small-scale agitation cyanidation.
Regardless of the leaching technology employed, the dissolved precious metals are recovered from the pregnant solution either by precipitation on zinc dust or by carbon adsorption-desorption-electrowinning. In larger scale operations, the precious-metal-bearing zinc precipitates and steel wool cathodes are fire-refined to obtain dore bullion. The dore bullion is either sold to a refiner or refined on site to obtain pure gold and silver bullion.
The operators of small mining properties in many instances cannot afford to construct a precious metal refinery and must sell their zinc precipitates or steel wool cathodes to a custom refiner. Marketing their product in this manner decreases the small operators profits because of loss of interest on the price of the metal while it is idle, assay charges, costs for packing, shipping, and insurance while in transit, and the refiners fees and profits.
The objective of this investigation was to develop a hydrometallurgical refining procedure to enable the smaller operator to produce high-purity precious metal products from zinc precipitates or steel wool cathodes. The chemistry used in this procedure is essentially the same as used for chemically refining jewelry or dental scrap.
Experimental Procedure
Gold- and silver-bearing zinc precipitate used in the experiments was obtained from a large operating gold mine. Analysis of the zinc precipitate was as follows:
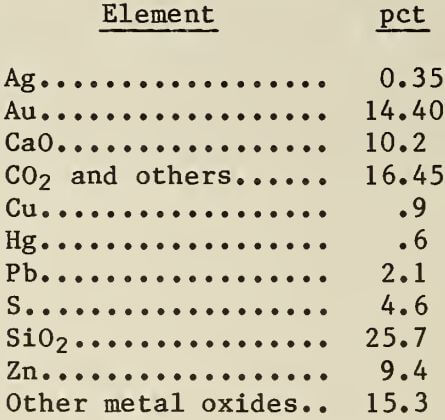
Analyses for specific elements and compounds contained in the zinc precipitate were made by fire assay, atomic absorption, and wet-chemical methods. Spectro-graphic analysis showed that the zinc precipitate contained small amounts of other metals.
The wet zinc precipitate was air-dried at room temperature for 120 h. The dry precipitate was sized on a 100-mesh screen. The oversize was ground to pass 100 mesh and mixed with the minus 100- mesh material in a glass bottle by rolling and tumbling.
A precious metal-bearing steel wool cathode was generated from the Bureau’s carbon stripping-electrowinning pilot demonstration unit (PDU) in Reno, NV. The cathode was oven-dried and weighed. Pieces were cut from different regions of the cathode and hand-blended to make a head sample. The sample was assayed by conventional fire assay methods. The steel wool cathode assayed 20.65 pct Au, 4.84 pct Ag, and 0.14 pct Cu.
Solid products (residues, Au sponge, AgCl precipitate, etc.) from all experiments were analyzed by fire assay for precious metal values. Solutions were analyzed by atomic absorption spectrophotometry for metal values.
Acid Pretreatment
Acid pretreatment experiments were conducted on 25-g charges of zinc precipitate which were agitated for 2 h at 80° C in 200 ml of 6M acid. Acids used were H2SO4, HCl, and HNO3. After leaching, the pulps were filtered, and the residues were washed with 100 ml of deionized water. Residues were dried, weighed, and analyzed for residual metal values.
A multistage HNO3 pretreatment experiment was conducted on a 25-g charge of zinc precipitate to improve silver recovery. The zinc precipitate was agitated for 2 h at 85° C in 100 ml of 6M HNO3. After pretreatment, the pulp was filtered and the filtrate was analyzed for silver and base metal content. The residue was leached three times. The final residue was washed with 400 ml H2O and was assayed for silver.
A 62-g steel wool cathode was digested at 90° C for 1 h in 1 L of 5M HCl to dissolve excess iron. The solids were separated from the acid solution by filtration. The solids were washed with distilled water, dried, and saved for aqua regia leaching to recover the contained gold values.
Precipitation of Silver
Silver precipitation experiments were conducted by adding known quantities of NaCl to slowly agitated silver-bearing HNO3 pregnant solution at room temperature. When initial AgCl precipitation ceased, 1 g of additional NaCl was added and the solution was gently agitated. If further AgCl precipitation was observed, additional NaCl was added in 1-g increments until precipitation was complete. The AgCl precipitate was separated from the acid solution by filtration, washed with distilled water, and dried.
Aqua Regia Dissolution of Gold
The gold-bearing residues from acid-preleaching zinc precipitates and steel wool cathodes (10 to 15.7 g) were agitated at 90° C for 1 h in dilute aqua regia. After leaching, the slurries were filtered on glass fiber filter paper and washed with distilled water.
A simulated two-stage aqua regia leaching experiment was conducted on an HNO3- leached residue. Ten grams of residue were agitated at 90° C for 1 h in 175 ml of dilute aqua regia. The pulp was filtered, and the filtrate was used to leach a fresh 10-g charge of residue at 90° C for 1 h. The slurry was filtered, and the washed residue was leached with dilute aqua regia.
Precipitation of Metallic Gold
One-hundred milliliters of gold-bearing dilute aqua regia solution was heated to 80° C. The stoichiometric amount of oxalic acid (0.69 g (COOH)2 per gram Au) required to precipitate the contained gold was added slowly and gently agitated. The solution was digested at temperature until gold precipitation stopped. Additional (COOH)2 was added to the solution in 1-g increments until precipitation stopped and the bright yellow color of the solution disappeared. The acid solution was decanted and allowed to stand overnight at room temperature for additional gold precipitate to form. The gold products were combined, washed with distilled water, dried, and assayed.
A second method for gold precipitation was to slowly bubble SO2 gas through a fritted glass dispenser into 100 ml of gold-bearing aqua regia solution at room temperature for periods ranging from 15 min to 1 h. The gold precipitate was washed with distilled water, dried, and assayed.
The third method evaluated was sodium bisulfite or sulfurous acid precipitation of gold from 100 ml of gold-bearing aqua regia solution. The sodium bisulfite (1.8 g NaHSO3 per gram Au) or sulfurous acid (2.5 g H2SO3 per gram Au) was added to the solution in one increment. The mixture was gently stirred for 15 min at 80° C. Precipitation was complete when no additional precipitation was observed and when the yellow color of the solution disappeared. The barren solution was analyzed for residual gold. The precipitate was filtered, washed, dried, and assayed.
Refining of Precipitated Gold
Separate charges of gold precipitated by (COOH)2 or by SO2 gas were mixed with nitre (KNO3), silica (SiO2), and borax (Na2 B4 O7) and heated in clay crucibles to 1,000° C for 1 h. The molten charges were poured into cast iron molds and cooled. The gold beads were fire-assayed for fineness, and the slags were fire-assayed for residual gold content.
In a separate experiment, gold precipitated with SO2 was upgraded in purity by digesting in 5M HNO3 at 90° C for 4 h. The gold sponge was washed with distilled water, dried, and assayed.
Cyanidation of Acid Leached Residues
for Recovery of Residual Precious Metals
Gold- and silver-bearing siliceous residue from aqua regia leaching of zinc precipitates (-7.5 g) was agitated at room temperature for 24 h in 100 ml H2O containing 0.3 to 1.0 g NaCN and 0.2 g CaO. The cyanide leached residues were filtered, washed, and dried.
Results and Discussion
The chemical technique for refining zinc precipitates differs from the process for steel wool cathodes. For refining zinc precipitates, the silver and base metals must be separated from the gold and silica. Since the steel wool cathodes contained no silica, separation of the gold from the silver by acid pre-leaching was not necessary. The residue generated from the aqua regia leaching of the HCl pretreated steel wool cathodes was a nearly pure AgCl product which assayed 74 pct Ag.
Acid Pretreatment
Preliminary tests showed that HNO3 was the only effective acid for dissolving the silver from the zinc precipitates. The results of HNO3 leaching experiments are shown in table 1. The data show that leaching with 6M HNO3 for 6 h dissolves 76 pct of the silver and most of the base metals and that multiple leaches are not justified.
HCl pretreatment of the steel wool cathodes generated an acid filtrate which contained 99 pct of the Fe, 28 pct of the Cu, 0.016 pct of the Au, and 1.7 pct of the Ag. The leached residue was 80.5 pct Au, 19.0 pct Ag, 0.4 pct Cu, and <0.1 pct Fe.
Precipitation of Silver
Silver can be precipitated as AgCl chloride from the HNO3 leaching solutions with NaCl according to the following equation:
NaCl + AgNO3 → AgCl(s) + NaN03……………………….(1)
The amount of silver contained in the HNO3 leaching solutions must be known to ensure the addition of the proper amount of NaCl. The results of NaCl precipitation of silver from HNO3 leaching solutions with stoichiometric and excess NaCl are shown in table 2. The silver was precipitated, but a large excess of NaCl was needed.
The dry AgCl precipitate is suitable for fire refining, or is marketable if it contains between 70 and 75 pct Ag.
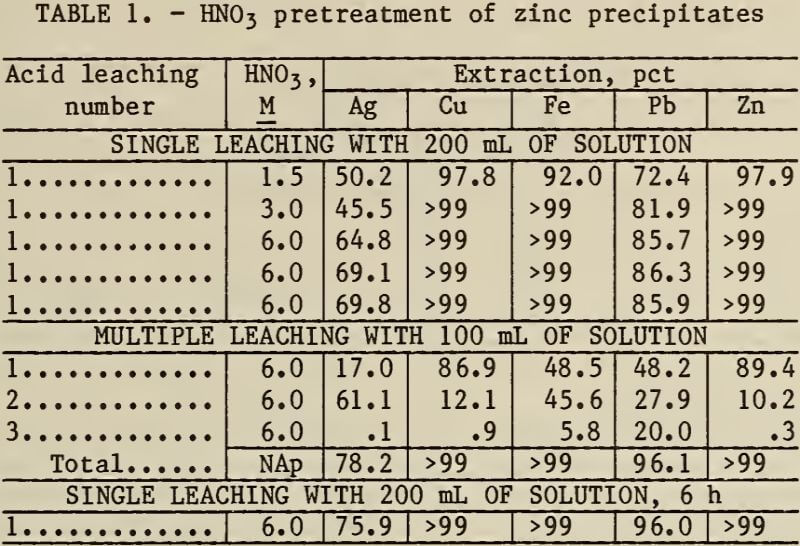
Note.—Leaching time = 2 h, multiple leaching totaled 6 h.
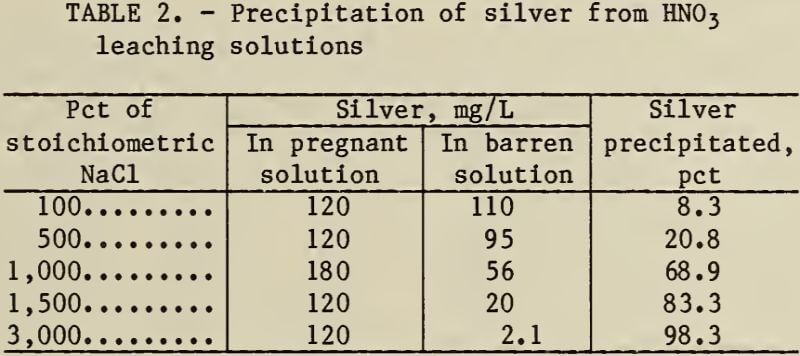
Aqua Regia Dissolution of Gold
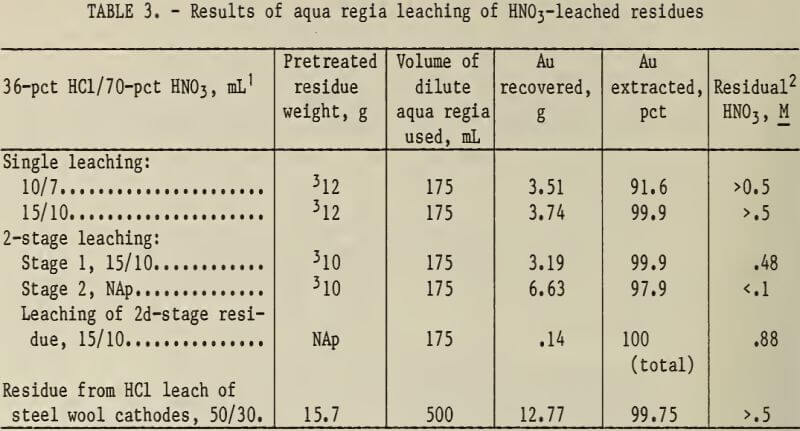
Dilute aqua regia leaching experiments were conducted to determine if gold could be extracted from residues from the HNO3 pretreatment of zinc precipitates and HCl pretreatment of steel wool cathodes. The amounts of HCl and HNO3 used were 150 to 300 pct in excess of the stoichiometric amounts required by the following equation:
Au + 3HNO3 + 4HCl → HAuCl4 + 3NO2 + 3H2O…………………….(2)
The experimental results are shown in table 3 and indicate that agitated leaching in hot dilute aqua regia dissolved metallic gold from the acid-leached residues.
A two-stage leach produced a pregnant AuCl3 solution low in residual HNO3, which improves the precipitation of gold from the aqua regia leaching solution.
Precipitation of Metallic Gold
(COOH)2 was added to the pregnant dilute aqua regia solution to precipitate gold as metallic crystals, according to equation 3:
3(COOH)2 + 2HAuCl4 → 2Au + 6CO2 + 8HCl………………………(3)
The experimental results for the chemical reduction of gold with (COOH)2 are shown in table 4.
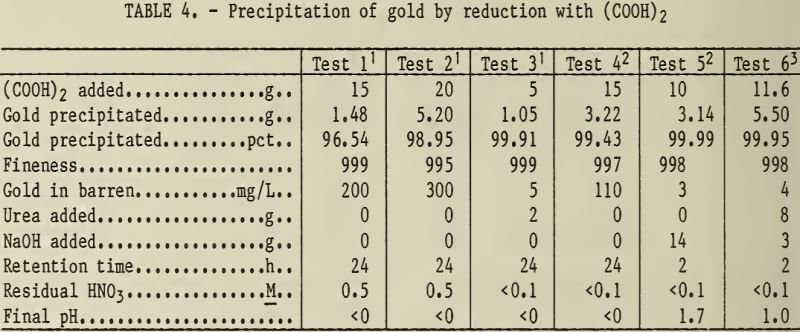
Note.—All tests made at 70° to 80° C.
The data show that reduction with (COOH)2 precipitated high-purity gold (998 to 999 fine) from dilute aqua regia leaching solutions. Five-tenths molar residual HNO3 in the leach solution resulted in slightly less precipitation of gold. Gold precipitation was higher from aqua regia leaching solutions from the second stage of the two-stage leaching experiment, which contained less than 0. 1M HNO3. Residual HNO3 could also be decreased by adding urea according to equation 4,
6HNO3 + 5CO(NH2)2 → 8N2 + 5CO2 + 13H2O…………………………(4)
increasing the percent of gold precipitated. Increasing the pH with NaOH
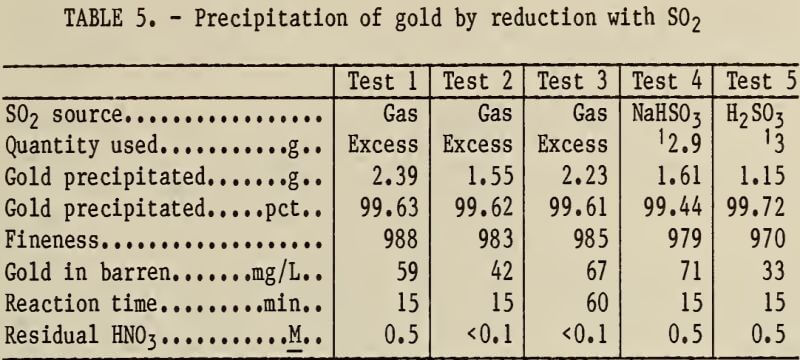
Note.—Pregnant solutions were generated by the aqua regia leaching of Zn precipitates. Tests using SO2 gas were carried out at room temperature (25° C). Tests using NaHSO3 and H2SO3 were heated to 80° C.
decreased the time required to precipitate the gold. The crystalline gold produced by (COOH)2 reduction is bright and lustrous.
SO2, NaHSO3, or H2SO3 was added to precipitate gold as metallic sponge, according to equation 5,
2AuCl3 + 3SO2 + 6H2O → 2Au + 6HCl + 3H2SO4………………………….(5)
The experimental results for the reduction of gold with SO2-producing compounds are shown in table 5. The gold precipitate was not as pure as gold precipitated with (COOH)2 and did not have the lustrous appearance.
Refining of Precipitated Gold
If the precipitated gold is not of high enough purity, refining by smelting or leaching with hot HNO3 may be necessary. Gold precipitated by SO2 was upgraded by digestion in hot HNO3 from 985 to 998 fine. The experimental results obtained from melting (COOH)2- or SO2- precipitated gold with nitre, borax, and silica are shown in table 6. Gold produced by chemical refining can be purified, if necessary, to 999.9 fine by fluxing with nitre, borax, and silica at 1,000° C.
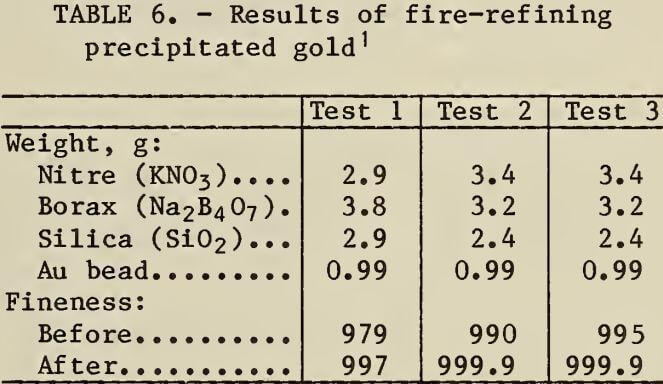
Cyanidation of Acid Leached Residues
for Recovery of Residual Precious Metals
The siliceous residues from leaching zinc precipitates contained residual precious metals. The results of cyanidation to recover the precious metal values are shown in table 7. Approximately 96 pct of the gold and silver in the residues was recovered by leaching in alkaline cyanide solutions. The precious-metal-bearing residues could be recycled to the plant’s cyanide leaching circuit.
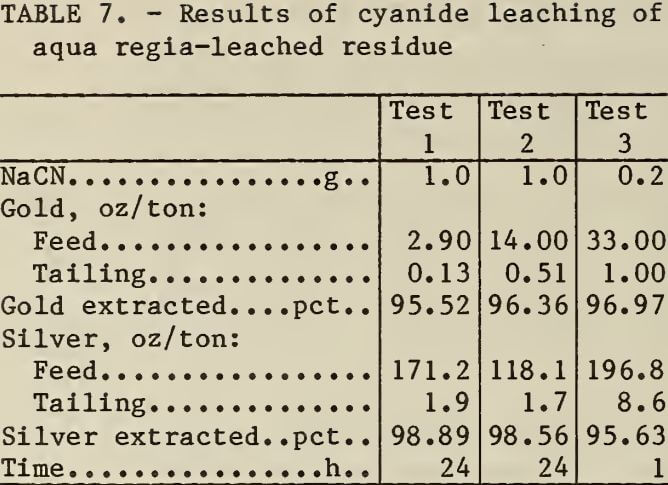
Slurry Solids for Acid Leaching Procedures
The slurry solids recommended for all the acid-leaching procedures are very low and depend on the amount of gold and/or silver contained in the specific feed. If the pulp solids are too high, the solubility limits for gold, silver, and other metals in the specific acid-leaching liquor may be attained, and pre-mature precipitation of precious metals and other metals may occur. The recommended pulp solids should be used unless it is determined by metallurgical testing that the slurry solids can be changed.
Recommended Procedure for Zinc Precipitates
The recommended flowsheets based on the results of this study are shown in figures 1 and 2. Acid pretreatment of zinc precipitates is required to dissolve the silver and base metals and leaves a gold-bearing siliceous residue. One kilogram
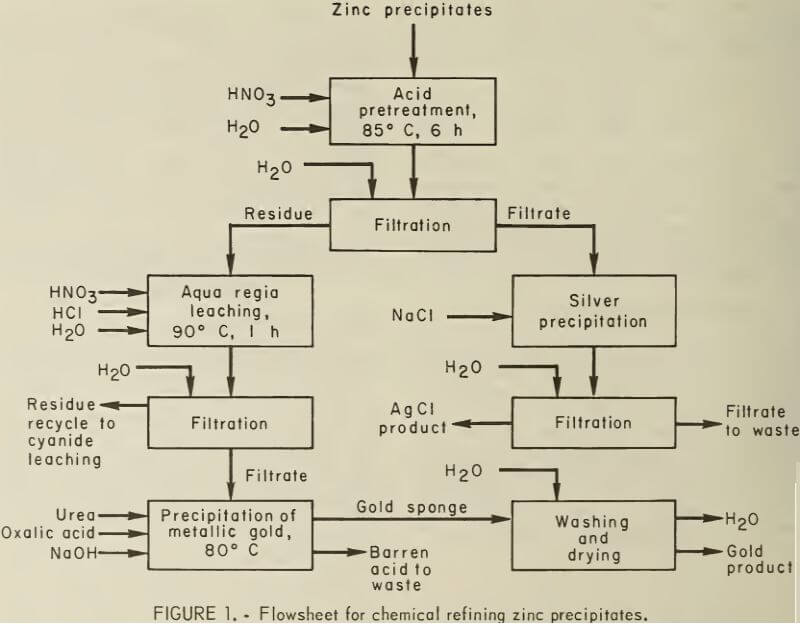
of zinc precipitate is slowly added to 10 L of 6M HNO3. The slurry is heated to 85° C, agitated, digested at temperature for 6 h, and filtered hot. The residue is washed with water. The wash water and acid filtrate are combined and saved for silver recovery.
The silver contained in the acid filtrate and wash water is precipitated as AgCl by the addition of NaCl. A tenfold stoichiometric excess of NaCl is added to the HNO3 leaching solution at room temperature. (The stoichiometric amount of NaCl is 0.54 g NaCl per gram of silver in solution.) The solution is agitated gently for 5 min and filtered. One gram of NaCl is added to the filtrate. If additional AgCl precipitation is observed, the above procedure is repeated. NaCl is added in the above manner until no precipitation is observed. In some cases a 30-fold excess of NaCl may be required. The AgCl product is washed with distilled water and dried at low temperature (<100° C).
Gold is recovered from the HNO3- preleached siliceous residue by leaching with aqua regia. The HNO3-leached residue is divided into two equal charges. One charge is slurried with dilute aqua regia. For every 100 g of charge, 2 L of dilute aqua regia containing 150 ml of 36.5-pct HCl and 100 ml of 70-pct HNO3 are used. The solution is heated to 90° C and digested with continuous agitation for 1 h. The slurry is filtered hot on glass fiber filter paper. The acid filtrate is removed, and the residue is washed with distilled water until no yellow color can be seen in the filtrate. The washes are combined with the acid filtrate. The first aqua regia-leached residue is saved for recycle to cyanide leaching. The acid filtrate is slurried with the second charge of HNO3-leached residue and treated as above. The second aqua regia leached residue is saved for further dilute aqua regia leaching.
Metallic gold is precipitated from the aqua regia leaching solution by (COOH)2. The pregnant AuCl3 solution (dilute aqua regia leaching solution) is heated to 80° C and treated as follows:
- Slowly add urea prills in 1-g increments until gas evolution stops. Add the stoichiometric amount of (COOH)2 required to precipitate the known or estimated amount of gold contained in the pregnant solution (0.69 g (COOH)2 per gram of gold in solution). Precipitation should start within 10 min. If precipitation does not start, or if it stops, add enough 30-pct NaOH solution to bring the pH to 1.0. Digest the solution at 80° C until precipitation stops.
- Add 10 pct of the previously calculated amount of (COOH)2. If precipitation is not observed, or if it stops, add enough NaOH to bring the pH up to 1.0. Digest the solution until precipitation stops. Repeat until the yellow color of the solution disappears. Combine the gold precipitate into a single sponge by using a spatula to free entrained gases and to scrape the sides and bottom of the vessel. When all of the gold has been collected, decant the barren solution and analyze for residual gold.
- Wash the gold sponge with distilled water and dry at 100° C. The dry gold sponge should assay between 997 and 999 fine.
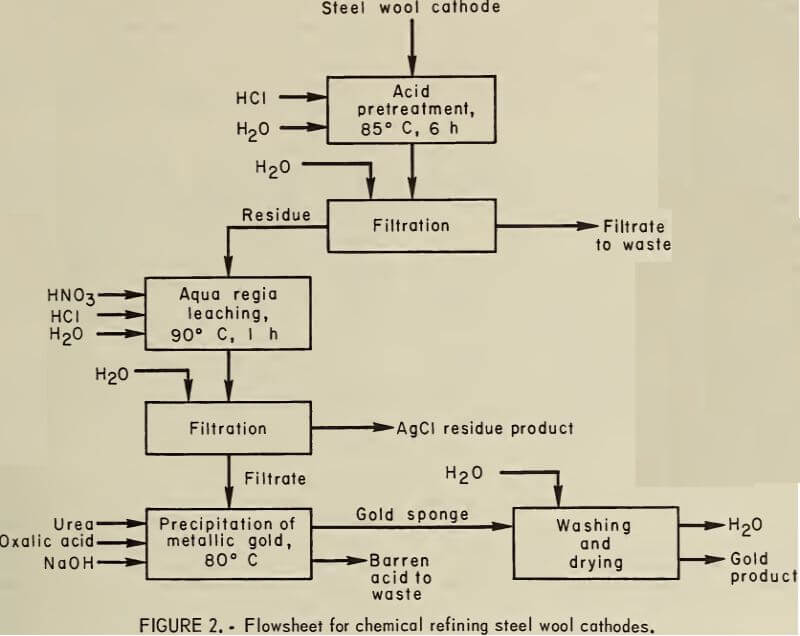
Recommended Procedure for Steel Wool Cathodes
HCl pretreatment of the cathodes is required to dissolve the steel wool. One kilogram of steel wool cathode is slowly added to 10 L of 5M HCl, which is heated to 90° C while stirring. The slurry is digested at temperature for 1 h, filtered hot, and washed with water.
The HCl-pretreated cathode residue is leached with dilute aqua regia to recover the gold and silver. The HCI leach residue is divided into two equal charges. One charge is slurried with dilute aqua regia. For every 100 g of charge, 2 L of dilute aqua regia containing 150 ml of 36.5-pct HCl and 100 ml of 70-pct HNO3 are used. The pulp is heated to 90° C, digested, and agitated for 1 h. The hot slurry is filtered on glass fiber filter paper, and the acid filtrate is removed. Displacement washing of the residue with distilled water is continued until no yellow color is visible in the filtrate. The washes are combined with the acid filtrate. The residue from the first aqua regia leach is an AgCl product which should contain 70 to 74 pct silver. The second charge of HCl-leached residue is slurried with the combined filtrate and treated as above. The second aqua regia- leached residue is saved for further dilute aqua regia leaching. The procedure for precipitating metallic gold from the resultant solution with (COOH)2 is the same as for zinc precipitates.
Handling and Disposal of Acid Solutions and Acid Wastes
The acid solutions used are corrosive and should be handled with extreme care. All leaching must be done in vessels that will not be corroded. The solutions should not contact the skin or eyes. Protective clothing should be worn to prevent accidental contact. Should the acid solution contact a person’s skin or eyes, flush with cold water for a minimum of 15 min and consult a physician.
The fumes from the acid solutions and chemical reactions with the solutions can be very hazardous. All operations should be conducted in a fume hood or in a well-ventilated area. If a fume hood is not available, ensure good outside ventilation and wear an appropriate chemical respirator and full protective clothing at all times.
The acid waste solutions should be neutralized with alkaline solutions before disposal. Violent reactions may occur when mixing acid solutions with alkaline solutions. Extreme care should be taken. The solutions should be slowly mixed to prevent an uncontrollable reaction. The pH of the neutralized acid waste should be between 6 and 7. The neutralized solutions should be disposed of according to local, State, and/or Federal regulations.
Zinc Precipitates & Steel Wool Cathodes by
Hydrometallurgical Refining of Gold
The experimental results showed that zinc precipitates and steel wool cathodes can be chemically refined to produce a pure metallic gold and an AgCl precipitate. Chemical refining of zinc precipitates and steel wool cathodes recovered 99.9 pct of the contained gold. HNO3 preleaching separated silver and base metals from the gold in zinc precipitates. Silver in the HNO3 leaching solution was recovered as AgCl by precipitation with NaCl. HCI leaching separated base metal from the gold and silver contained in steel wool cathodes. Silver was recovered as an AgCl precipitate which contained some gold.
Aqua regia leaching dissolved gold in the residues from HNO3 and HCl preleaching. (COOH)2 and SO2 precipitated metallic gold from the aqua regia leaching solutions. The gold produced by (COOH)2 reduction was 998 to 999 fine. Gold produced by SO2 reduction was 970 to 988 fine, but was upgraded to 998 fine by digestion in HNO3. The gold can be purified to 999.9 fine by fire refining.
