Our Hydrogen Gas experiment involves taking one or two grams of zinc, put them in a test-tube, and add a few drops of dilute sulphuric acid; an effervescence takes place, and bubbles of gas rise through the liquid. Put a lighted taper into the test-tube ; a slight explosion takes place, and you see a momentary flash.
The following equation explains the reaction that takes place:
Zn + H2SO4=ZnSO4 + H2
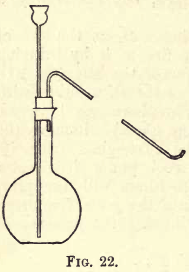
To prepare hydrogen for experiments, take a flat-bottomed flask of about 300 c.c. capacity and fit it up with a thistle funnel and delivery tube as shown in fig. 22. Slide carefully into the flask about 30 grams of granulated zinc, inclining the flask to one side while so doing to prevent the zinc from striking heavily on the bottom of the flask, and perhaps cracking it; fit the tube and funnel into the flask, and pour through the funnel sufficient water to just cover the zinc. Fix the flask now on a retort stand with the delivery tube dipping into the pneumatic trough, as in the preparation of oxygen, and get two gas jars ready as before. Add, little by little, dilute sulphuric acid through the funnel, just sufficient to keep up a steady effervescence. Allow time for the air to be displaced in the flask, then collect some of the gas in a test-tube; when full place the thumb under the mouth of the test-tube while under the water and remove it from the trough. Keep the test-tube mouth downwards, remove the thumb and apply a light; if an explosion takes place, the gas is still mixed with air ; so repeat the experiment until no explosion takes place, then the gas is ready to collect in the jars. Collect two jars full of the gas, and use cover-glasses as before.
Hydrogen Gas Laboratory Experiment I
Take a jar of the gas from the trough, and, holding it mouth downwards, remove the cover-glass and apply a light to the mouth of the jar; the gas takes fire and burns with a non-luminous flame; pass the taper further up into the jar and it is extinguished. You will observe that under ordinary circumstances hydrogen is flammable, but does not support combustion like oxygen.
Hydrogen Gas Laboratory Experiment II
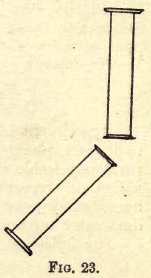
Take the other jar of the gas from the trough, keep it inverted in the right hand, while in the left you hold, also mouth downwards, a jar containing air only; pour upwards, as it were, the hydrogen gas into the jar containing the air (see fig. 23), in a few moments all the hydrogen will have passed into the upper jar. Set the jar from which you have poured the gas mouth downwards in the trough and bring a lighted taper to the mouth of the other jar, still keeping it inverted; a slight explosion follows, and then you see the pale non-luminous flame of burning hydrogen. Now take the jar from the trough and apply a light to its mouth; no similar result follows, showing that all the hydrogen has been poured out. This experiment proves the lightness of hydrogen gas.
Filter the liquid remaining in the flask containing the zinc and sulphuric acid, and evaporate it down to about one-third its bulk in a porcelain basin, then set it aside to cool; white crystals of zinc sulphate will be found to separate out.
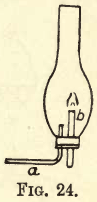
The relativity of the terms ‘ combustion ’ and ‘ supporter of combustion ’ may be shown with gases other than pure oxygen and hydrogen. Air, for instance, may be made to burn in an atmosphere of coal gas. Take an ordinary lamp cylinder and fit it with a cork and glass tubes as shown in fig. 24. Attach a to a gas jet and send a current of coal gas into the cylinder; allow time for the cylinder to be completely filled with coal gas, then pass a lighted taper up the open wide tube b; a bluish flame is formed, and will continue to burn as long as the current of coal gas is kept passing through the cylinder.
In this experiment you see that a gas which, under ordinary circumstances, is combustible, can apparently be converted into a supporter of combustion.
